Applying for provisional registration extension or transition to full registration
Guidance on obligations during provisional registration period, process for applying for extension of provisional registration and transition to full registration.
Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is for sponsors of provisionally registered prescription medicines. It describes the post-market obligations during the provisional registration period as well as the legislative requirements and processes for applying for extensions of provisional registration and transition to full registration.
To assist with the process of applying for provisional registration, see:
Provisional registration period
Lapsing of provisional registration
The provisional registration period is the 2 years starting on the day registration commences (section 29(3) of the Therapeutic Goods Act 1989 (the Act)).
Your provisional registration will automatically lapse at the end of this period, unless:
- you have made an application for an extension to the provisional registration period and we have granted it (section 29(6) of the Act), or
- we have extended the period of provisional registration while considering an application for full registration (section 29(10) of the Act)
You cannot appeal automatic lapsing of provisional registration under the provisions of section 60 of the Act as lapsing is not a regulatory decision.
We expect that you will inform health professionals and patients of any decision not to extend provisional registration prior to lapsing given that provisionally registered medicines are brought to market earlier for unmet clinical needs.
Where appropriate, patients may be able to continue accessing a medicine after provisional registration has lapsed through existing avenues for unapproved therapeutic goods.
If reapplying for provisional registration following lapsing, you must first submit a new provisional approval determination application with the applicable fee followed by a new provisional registration application.
Requirements in the provisional registration period
You are required to comply with the relevant conditions of registration which were imposed at the time of registration, similar to other medicines.
The need for any Australia-specific requirements will be considered during the pre-market registration process, and you will be advised through the delegate's overview.
Collection of confirmatory data on efficacy and safety
To maintain provisional registration, you must continue to demonstrate that you can provide confirmatory data on efficacy and safety before the end of the provisional registration period. This includes:
- completing confirmatory studies as part of your Risk Management Plan (RMP/ASA) which is imposed as a condition of provisional registration. Key milestones and completion dates will be specified as a condition of the provisional registration
- continually assessing the benefit-risk of the provisionally registered medicine
To maintain provisional registration, the benefit-risk profile of the medicine must be positive and this must be maintained throughout the period of provisional registration to the transition to full registration.
The collection of confirmatory data on safety and efficacy should lead to submission of a Category 1 Type S application for transition to full registration. The initial approval for provisional registration imposes a condition of provisional registration that determines when the data is expected to be received by the TGA. The due date is based on the clinical study plan submitted by the sponsor with the initial application for provisional registration and must be submitted before the end of the maximum 6 years that start on the date that initial provisional registration commenced (noting that the initial provisional approval period is 2 years).
The clinical trial plan can only be amended following TGA approval of the change. An amendment to the clinical trial plan does not result in an extension of the provisional registration period, which remains a maximum of 6 years from the date of the initial provisional registration. If circumstances require an update to the clinical trial plan, this should be submitted to the TGA for approval, for example as part of an update to the clinical trial plan submitted with an extension of provisional registration application, or other related category 1 application.
Enhanced post-market monitoring
You are required to comply with the existing post-market requirements set out in our Pharmacovigilance responsibilities of medicine sponsors. In addition, the normal requirements for submitting RMP updates after regulatory approval apply.
We will determine additional post-market requirements on a case-by-case basis. These may include consideration of whether specific safety concerns could be best addressed using data collected from an Australian patient registry.
We may also require you to ensure that information about provisionally registered medicines is clearly communicated to patients and health professionals.
Provisionally registered medicines will be given high priority for post-market surveillance activities under our enhanced post-market monitoring and compliance framework.
Enhancements to this framework include:
- an RMP Compliance Monitoring Program
- the Black Triangle Scheme and
- a Pharmacovigilance Inspection Program
All provisionally registered medicines including those with a combination of provisionally and fully registered indications will be included in the Black Triangle Scheme.
Periodic Benefit-Risk Evaluation Reports (PBRERs) are usually submitted annually for the first three years of registration. We will determine whether a PBRER, or suitable alternative, is required more frequently, for example 6 monthly, for a provisionally registered medicine. This will also be required for a longer period than the standard 3 years to account for the provisional registration period, which may last up to 6 years. Where possible, reporting requirements and timeframes will be aligned to those required by the European Medicines Agency (EMA).
Provisionally registered medicines may also be selected for targeted or batch release laboratory testing. This may require submission of samples, reference standards, internal controls and, if required, proprietary reagents or cell-lines.
TGA initiated variations for provisionally registered medicines
The Secretary can vary an entry in the ARTG in relation to a provisionally registered medicine where it appears that the quality, safety or efficacy of the medicine is unacceptable in relation to a class of persons (subsection 9D(1A) of the Act).
TGA initiated variations are limited to those that will:
- reduce the class of persons for whom the medicine is suitable (for example excluding children or the elderly from the relevant class);
- change the directions for use of the medicine; or
- add a warning or precaution to the medicine
The Secretary may also vary the PI relating to the medicine (subsection 25AA(4) of the Act) if either:
- under section 9D, the Secretary varies the entry in the Register in relation to the medicine
- there is a change in the conditions to which the inclusion of the medicine is subject; and as a result of that variation or change, the Secretary is satisfied that a variation to the product information is appropriate and reflects the basis on which the Register has been varied or the conditions have been changed.
We will inform you in writing of the intent to make the variation and the reasons for it. We will provide you with reasonable opportunity to respond and will take your responses into account before making the final decision to vary the ARTG.
Sponsor initiated variation for provisionally registered medicines
Sponsors of provisionally registered medicines may need to vary aspects of their medicines as part of their product development, while they are still provisionally registered.
A number of these kinds of variations (e.g. a different dosage form or strength) result in the creation of a separate and distinct good.
No requirement for provisional determination
To avoid delay in access to promising new medicines a new provisional determination is not required prior to application lodgement where the following applies:
- there already is a medicine that is provisionally registered in the Register because of an application that, under subsection 23AA(1) of the Act, was taken to be an application for provisional registration - this is the original medicine on which the new medicine is based; and
- another medicine - the new medicine - is separate and distinct from the original medicine, under subsection 16(1) of the Act (e.g. if they have different directions for use); and
- the sponsor applies under section 23 of the Act for the registration of the new medicine, before the end of the original medicine's provisional registration period;
- the sponsor specifies in the application that they are seeking the provisional registration of the new medicine; and
- at the time of that application, the active ingredients, and indications of the new medicine and the original medicine are the same the application may not extend the indications of the medicine (i.e. can't be a new indications medicine).
If all requirements for sponsor-initiated variation are met, an application made under section 23 AA (2) of the Act in relation to the new medicine will be taken to be an application for the provisional registration, with no requirement for the sponsor to first obtain a provisional determination under the Act in relation to the new medicine.
Variation applications under section 9D of the Act do not require provisional determination.
You are required to seek provisional determination for applications to provisionally register new prescription medicines and new indication medicines (Therapeutic Goods Regulations 1990).
- The determination needs to be in force prior to lodgement of an application under section 23 through the relevant form.
Application types and fees for variation of provisional register entries
The same application types and fees that apply to registration applications for variation under the standard process apply to variations of provisionally registered medicines.
Note that distinct fees apply to applications to register new prescription medicines, new indication medicines, extensions of provisional indication and transition from provisional registration to full registration.
Grouping of applications
The Therapeutic Goods (Groups) Order No. 1 of 2001 ("the Grouping Order") permits the grouping of certain registered goods, including provisionally registered goods, as gazetted therapeutic goods groups in accordance with section 16 of the Act. The existing grouping order will continue to allow applicants to combine for example, new directions for use under an existing ARTG entry.
How to apply
Applicants should carefully consider above requirements before submitting provisional variation applications to avoid the loss of application fees. The Act contains no provisions to refund application fees to applicants after the application has been received by the TGA.
Lodge applications for sponsor-initiated variation by providing a completed Application Form and the required supporting documentation. Depending on the application type, different application forms are required and lodged either through the TGA Business Services (TBS) website, or via email. Further guidance on what form to use and how to lodge is available on the TGA website.
If the application is made under subsection 23AA of the Act please ensure that the application form specifies the application is for provisional registration:
- The TBS variations e-form - provides a free text comment box that should be used to specify that the application is for provisional registration.
- The Module 1.2.1 Application form to register or vary the registration of prescription medicines provides a check box in Part B, submission details
If approval of the requested variation under subsection 23AA would impact on multiple ARTG entries, you should list all ARTG affected entries in the application form.
Product Information (PI) and Consumer Medicines Information (CMI)
You must include a statement in the PI and CMI explaining that the medicine or indication is provisional. These statements must be approved by the TGA and also appear in all relevant promotional and educational material for the provisionally registered medicine.
PI statement
A statement based on the template suggested below must be included in 'section 4.1 Therapeutic Indications' of the revised PI format. The exact wording will vary on a case-by-case basis, depending on the medicine and the evidence. The term provisional approval should appear in bold. If a medicine has a combination of provisional and non-provisional indications, list the provisional indication first. The statement must be included with each provisionally registered indication.
This medicine has provisional approval in Australia for the treatment of (insert TGA approved wording for indication). The decision to approve this indication has been made on the basis of (insert endpoints that supported provisional approval and major study limitations). Continued approval of this indication depends on verification and description of benefit in confirmatory trials.
CMI statement
Include the following CMI statement below the name of the provisionally approved medicine in the CMI document. The term provisional approval should appear in bold. If the medicine has a combination of provisional and non-provisional indications, insert only the provisional indication within the statement.
This medicine has provisional approval in Australia for (insert indication). The decision to approve this medicine has been made on the basis of promising results from preliminary studies. More evidence is required to be submitted when available to fully confirm the benefit and safety of the medicine for this use.
Tracking and enforcement of provisional registration
Tracking compliance to RMP requirements
The RMP Compliance Monitoring Program will proactively follow up on RMP commitments, such as additional pharmacovigilance and risk minimisation activities.
If it appears that high priority activities have not been completed within agreed timeframes, we will notify you in the first instance and request a response. This will enable early identification and remediation of non-compliance.
If our attempts to work with you to achieve compliance are unsuccessful, we will consider taking regulatory action.
Direct enquiries relating to RMP compliance monitoring to rmp.coordinator@health.gov.au
Cancellation, suspension and penalties
To maintain patient safety, we can suspend or cancel provisional registration if there is evidence that the benefit-risk balance of the product has changed (for example where a trial does not verify efficacy).
Existing enforcement powers under section 29 and section 30 of the Act apply to provisionally registered medicines.
Penalties, cancellation or suspension of provisional registration may occur if you refuse or fail to comply with a condition of provisional registration or if it becomes apparent that you will be unable to obtain the confirmatory data required to obtain full registration, for example, if a pivotal trial in the clinical trials plan is unable to recruit, or has been stopped or halted.
Following consultation with you, we reserve the right to revoke provisional registration if at any time an issue emerges regarding the safety and efficacy of the medicine.
We will process and publish sponsor-initiated requests for cancellation of provisional registration as per the current process and legislative requirements for requesting cancellation of an ARTG entry.
Communication of provisional registration
TGA communication on provisionally registered medicines
TGA is committed to providing clear information to consumers and health professionals that a medicine has been granted provisional registration and the implications of this for health professionals, patients and their carers.
We will publish medicines that have received provisional determination on our Determinations and designation notices web page.
We will also publish high-level information on your progress towards completing pivotal studies during the provisional registration period. This may include a list of confirmatory trials and their status, i.e. ongoing, halted, complete etc.
At times we may publish additional information regarding provisionally registered medicines through currently available channels such as updates on safety information, news and media releases.
Sponsor communication on provisionally registered medicines
You may be required to undertake additional risk minimisation and communication activities. This will be considered on a case-by-case basis.
Communication activities may include patient and/or health professional education, Dear Healthcare Professional letters or limitations on which health professionals can prescribe the product (such as controlled access schemes).
These commitments will be detailed in your RMP and will fall into the scope of the RMP compliance monitoring program.
Annual charges and fees for provisionally registered medicines
The current fees for different applications in the provisional pathway are set out in Schedule 9 of the Therapeutic Goods Regulations 1990 (the Regulations).
The activities required in the provisional registration period will be cost recovered via higher annual charges and / or higher fees, where required.
Industry consultation will be conducted prior to implementation of any changes to fees and/or charges.
Extension of provisional registration
This guidance is for sponsors of provisionally registered prescription medicines who are seeking an extension to their provisional registration on the Australian Register of Therapeutic Goods (ARTG).
You may apply for an extension of the initial provisional registration period (section 29(4) of the Act). Up to two extensions of up to 2 years each are available, resulting in a possible maximum provisional registration period of 6 years. These regular checkpoints during the provisional registration period allow us to assess if you are on track with fulfilling post-market conditions.
The provisional registration extension process
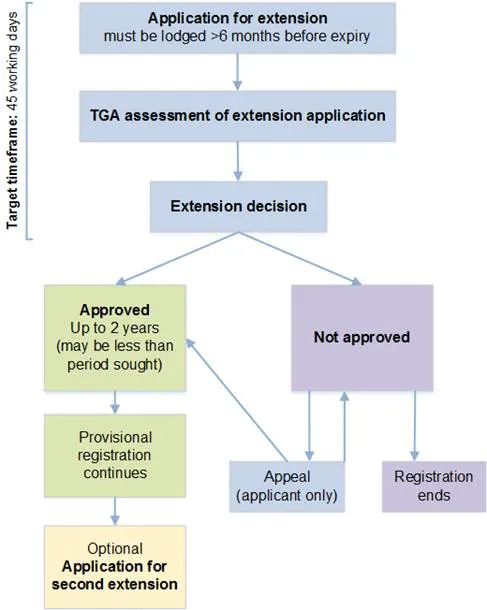
The flowchart illustrates the our provisional registration extension process, with a target timeframe of 45 working days. The process follows these steps:
- Initial Stage:
- Applications must be lodged more than 6 months before the expiry date
- TGA conducts an assessment of the extension application
- An extension decision is made
Decision Outcomes:
If Approved:
- Extension granted for up to 2 years (may be less than period sought)
- Provisional registration continues
- Optional application for second extension available
If Not Approved:
- Registration ends
- Applicant-only appeal process available
- Appeal Process:
- Available only to applicants
- Can result in either:
- Approval (leading back to the approval pathway)
- Rejection (leading to registration end)
The flowchart uses colour coding (light blue for process steps, green for approval pathway, purple for rejection pathway, and beige for optional steps) and directional arrows to show the relationship between steps. Each decision point and outcome is clearly marked with connecting arrows showing possible pathways.
When to apply for an extension of provisional registration
You must submit an application for extension of provisional registration at least 6 months before the provisional registration is due to end (refer subsection 29(5)(c) of the Act).We will not accept applications for extension of provisional registration which are submitted later than the 6 month cut-off date.
The provisional registration period will automatically lapse at the end of the current provisional registration period if you do not lodge an application for extension (or an application to transition to full registration).
Requirements when not applying for an extension
Notify us in writing as soon as you have decided that you do not intend to apply for an extension of provisional registration. This allows us to provide timely communication to patients and healthcare professionals that the provisional registration will lapse and therefore the medicine will no longer be included in the ARTG. This communication is likely to be provided though an update on the TGA website.
We expect that you will inform health professionals and patients through Dear Healthcare Professional letters and other channels of communication.
You can make this notification by sending an email to Aet.Application.Entry.Team@health.gov.au with the following details:
- details of the medicine (trade name, ingredient name, ARTG entry number)
- sponsor name
- expiry date of provisional registration
- current approved indication for provisional registration
- reasons for not applying for ongoing provisional registration (for example voluntary market withdrawal, safety concerns, or delays in the clinical trials plan)
Step 1: Submitting your application for extension
You must:
- submit an application for extension of provisional registration according to the requirements outlined in subsection 29(5) of the Act.
- submit an application for extension in an approved form accompanied by the application fee at least 6 months before the provisional registration is due to end
- provide all the information required in the form including additional documentation as described below.
It is your responsibility to ensure that the extension application form, application fee and required documentation have been lodged 6 months before lapsing of provisional registration.
Fees and refunds
The application fee for extensions of provisional registration can be found in our schedule of fees and charges.
You may be eligible for a refund or waiver of the application fee if you have a valid Orphan drug designation at the time of submitting your application. See Apply for a prescription medicine via orphan drug designation.
Documentation required to support your extension application
You must include the following with your application to extend provisional registration:
- evidence of a valid Orphan drug designation if applicable
- an updated clinical study plan
Email the required application form and required documentation as an electronic submission to esubmissions@tga.gov.au CC AET.Application.Entry.Team@health.gov.au selecting Extension of provisional application [T] in the form.
Clinical data for provisional registration extension
The application for extension of provisional registration should demonstrate the progress of pivotal studies to verify clinical benefit against the milestones detailed by the clinical study plan. We will assess the current status of confirmatory trials. If additional data has been specified in the study plan, you will need to provide an update on this.
You are required to demonstrate progress of the clinical trials against the agreed milestones and timeframe of the agreed clinical study plan.
The following aspects should be considered:
- progress against milestones set out in the clinical study plan
- changes in study milestone(s)
- whether we have agreed to changes in study milestone
- The framework for submission of updates to the clinical study plan and confirmatory study results will be made available at a later stage.
Step 2: TGA assessment of your extension application
Your application for extension of provisional registration should demonstrate the progress of pivotal studies to verify clinical benefit against the milestones detailed by the clinical study plan.
On receiving the application, the Secretary must decide to grant, or to refuse to grant, an extension of the provisional registration period (section 29(6) of the Act).
In making that decision the Secretary must consider:
- whether they are satisfied with your (the applicant's) plan to submit comprehensive clinical data on the safety and efficacy of the medicine before the end of the 6 years starting on the day the provisional registration commenced, and
- other matters (if any) as the Secretary considers relevant
We will assess:
- your progress towards submitting comprehensive clinical data on the safety and efficacy of the medicine before the end of the provisional registration period
- any new information on safety data
- any other matters submitted or which we deem to be relevant.
We may ask for more information and seek expert advice as required during the assessment of an extension application.
Timeframes for assessment of extension applications
We will make a decision within a target time frame of 45 working days on receiving a complete application for extension of provisional registration. Any time taken for you to respond to requests for additional information does not count towards the target timeframe for our assessment.
We will provide you a written notice of the extension decision as soon as practicable after making the decision.
The assessment and decision-making timeframe does not commence until the extension fee has been paid (if applicable).
Step 3: Notifying sponsors of the extension decision
The decision to grant or refuse to grant an extension of provisional registration is a formal decision under section 29(6) of the Act.
If we decide to extend the provisional registration period, the decision letter will specify the period of the extension. This period must not exceed two years and may be less than the period sought.
If the extension is not granted, the reasons for refusal will be provided in our decision letter.
If you wish to appeal this decision, you have up to 90 calendar days (commencing on the day on which the decision was made) to lodge an appeal under section 60 of the Act. Note that only the sponsor of the relevant provisionally registered medicine is able to appeal this decision.
At the time of granting an extension, we may also impose new conditions of provisional registration or vary the existing conditions. These new conditions will be communicated in the decision letter and if relevant, should be included in a revised RMP/ASA.
New conditions may be imposed, for example, if an anticipated clinical trial does not proceed or the design of a relevant trial is significantly changed so that the clinical development of the medicine for an identified class of persons does not proceed. In this case, the Secretary may decide to vary the ARTG entry to exclude this identified class of persons from persons for whom the medicine is suitable.
Transition to full registration
This guidance is for sponsors of provisionally registered prescription medicines. It assists sponsors with the process of applying to transition a provisionally registered medicine to full registration on the Australian register of Therapeutic Goods (ARTG).
This guidance should be read in conjunction with the standard prescription medicines registration process because the elements for transition to full registration are similar.
Overview of the transition from provisional to full registration
You can lodge your application to transition to full registration at any time up until the provisional registration lapse date, however, you should not apply for full registration until you have completed the obligations outlined for the provisional registration period and complete confirmatory data on safety and efficacy are available.
You will need to make a formal application under section 23 of the Act to seek full registration of the medicine. Your submission for full registration may also include applications for broader indications than the provisionally registered indication.
If your application is approved, it will then be considered 'fully registered' for the purposes of the ARTG (section 29(9) of the Act).
You do not need to apply for an extension of provisional registration (if eligible) if you are seeking full registration, as the provisional registration period continues while we are considering your application (section 29(10(b)) of the Act)
In this circumstance, the provisional registration period may extend beyond the specified maximum 6 years. This will allow you to continue to supply the medicine while we are evaluating your application for full registration.
Requesting priority review for transition to full registration
You may request priority review of your application for transition to full registration. You must first apply for priority determination and meet the eligibility criteria for priority review. See
If a priority determination application is lodged and approved, your application will be processed via the priority review registration process.
Requirements when not applying for full registration
Notify us as soon as possible if you do not intend to apply for full registration. This allows us to provide timely communication to patients and health professionals that the medicine will be removed from the ARTG. This communication is likely to be provided though an update on the TGA website.
We also expect that you will inform health professionals and patients through Dear Healthcare Professional letters and other channels of communication.
You can make this notification by sending an email to AET.Application.Entry.Team@health.gov.au with the following details:
- details of the medicine (trade name, ingredient name, ARTG entry number)
- sponsor name expiry date of provisional registration
- current approved indication for provisional registration
- reasons for not applying for full registration (for example voluntary market withdrawal, safety concerns, delays in clinical trials plan
Phase 1: Requesting a pre-submission meeting
You are not required to arrange a pre-submission meeting if you are seeking transition from provisional registration to full registration.
Phase 2: Submitting your application for full registration
The application follows the standard prescription medicine registration process and includes Pre-submission form lodgement and Submission lodgement through electronic submissions.
Timeframes for assessment
The statutory timeframe is 255 working days (regulation 16C of the Therapeutic Goods Regulations 1990). However, we will prioritise the evaluation of applications for transition from provisional to full registration within this legislated time frame.
Application and evaluation fees
See our schedule of fees and charges for application and evaluation fees for an application to transition from provisional registration to full registration.
The timing and mechanism for payment of these fees is the same as the standard prescription medicines registration process.
Documentation required for your application
We will not accept rolling submissions of clinical data during assessment for full registration. However, new safety data is acceptable during the evaluation process.
You may cross-reference information previously submitted during the provisional registration period in the dossier for full registration which must be submitted in eCTD. We also require you to provide:
- cross-reference to the related provisional registration applications
- if applicable, evidence of a valid Orphan drug designation or Priority review determination
- evidence of having met your RMP obligations. This can be a chronological listing of your RMP obligations during the provisional period outlining completion of conditions. It should include dates when data were submitted and reasons for delays or failure to meet obligations
Submitting results of confirmatory trials
Include all final results not previously submitted from confirmatory trials in the dossier as per the current requirements for registering a prescription medicine. Clinical trials data must support the indication in the application for full registration.
Depending on the nature and results of the confirmatory trials, the indication which is subject to an application for full registration could be narrower or broader than the provisionally registered indication.
Phases 3 to 6: First round assessment to Expert advisory review
Phases 3, 4, 5 and 6 are the same as the standard prescription medicines process.
Phase 7: Notifying sponsors of the decision on full registration
The Secretary will approve the medicine to be registered on the ARTG where they are satisfied that the benefits sufficiently outweigh the risks, as for any submission to register a prescription medicine.
For applications to transition from provisional to full registration, however, the Secretary can now make a decision about the provisionally registered medicine under section 29(9) of the Act.
This is in addition to the existing authority under section 25 of the Act to decide to register the product on the basis of efficacy, safety and quality. Section 29 (9) allows the Secretary to make decisions in relation to ending or extending the provisional registration if required.
The Secretary may exercise several options:
- allow provisional registration of that portion of the indication for the medicine to continue until it expires;
- extend the provisional registration for a further period of the Secretary's choosing, up to a maximum of 6 years after the provisional registration commenced; or
- end provisional registration at the same time as the decision not to register the medicine, due to safety concerns
The Secretary's decisions and reasons for these decisions will be provided in the decision letter to the sponsor.
The decision letter will also set out the appeal rights. Decisions relating to applications to transition to full registration are subject to appeal by the sponsor only under section 60 of the Act.
Step 4: Post–decision activities
You are responsible for lodging an updated PI and CMI with TGA after your application is approved for transition to full registration.
We will outline any additional communication activities and post-market requirements in our decision letter.
Example clinical study plan for provisional registration extension applications
Example overview of conditions of registration imposed and fulfilment to be provided at the time of submitting an application for extension of provisional registration
Example clinical study plan for provisional registration extension applications (docx,36kb)
| Description of condition of registration in decision letter | Due date | Type of condition | Scope | Description of the approved change in scope | Reason for the change in scope (if applicable) | Status (including timeliness) and updated due date | Reason for delay (if applicable) |
|---|---|---|---|---|---|---|---|
| e.g. Overall Survival follow up included in study xxx should be provided as a category 1 application, including sub-analysis of 'specified patient category'. The data should be presented in the context of historical controls. 'Source of reference' until 'year' or when the overall survival data is sufficiently mature (at least 50% OS events observed), whichever occurs earlier. | DD/MM/YYYY | Clinical study | Unchanged/ Changed in scope approved as part of application for minor/major variation | Ongoing on track / Ongoing delayed Completed on track Trial halted |
Supporting documents
Page history
Update to include provisional variation for existing provisional entries.
Update to PI statement
Minor formatting changes to PI and CMI statement
Update to PI statement
Original publication
Update to include provisional variation for existing provisional entries.
Update to PI statement
Minor formatting changes to PI and CMI statement
Update to PI statement
Original publication

