Understanding the process of submitting priority inclusion application
Guidance to help sponsors understand the process of submitting a priority inclusion application. This is for sponsors of biologicals with (priority applicant) determination.
Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is to assist sponsors in understanding the process for submitting applications for priority inclusion of biologicals in the Australian Register of Therapeutic Goods (ARTG).
It outlines the key differences between the priority and standard biological inclusion pathways and should be read in conjunction with the user guide biologicals application form - A step-by-step guide.
Legislation
Priority and standard inclusion pathways
The standard and priority inclusion pathways both consist of 8 phases. However, the priority pathway includes modifications to reduce timeframes:
- the priority inclusion pathway has greater flexibility between phases, which allows the application to progress to the next phase more quickly
- you will receive rolling questions during the evaluation phase. If you can respond to all rolling questions by the end of the first round of evaluation a stop clock will not be applied, and the evaluation can proceed to the next phase.
- there are more flexible arrangements for accessing expert advice.
As per the standard pathway, we will provide you with updates to the evaluation plan to reflect any changes in timeframes.
Priority inclusion timeframes
The priority inclusion pathway is designed with a target timeframe of 150 working days.
In comparison, the standard inclusion pathway aims to process submissions within a target timeframe of 255 working days.
The above timeframes are calculated from acceptance of your application for evaluation through to our decision.
You are responsible for providing us with all necessary information in a timely manner. If you cannot meet this requirement, we may convert your priority submission to the standard pathway. For more information see Exit criteria.
Priority inclusion process
The following flowchart provides an overview of the priority inclusion process. Please note however, that not all steps may be necessary for every priority review application.
The timeframes in the flowchart represent the maximum timeframes for each phase of the process and are offered as a guide only.
Priority inclusion process overview
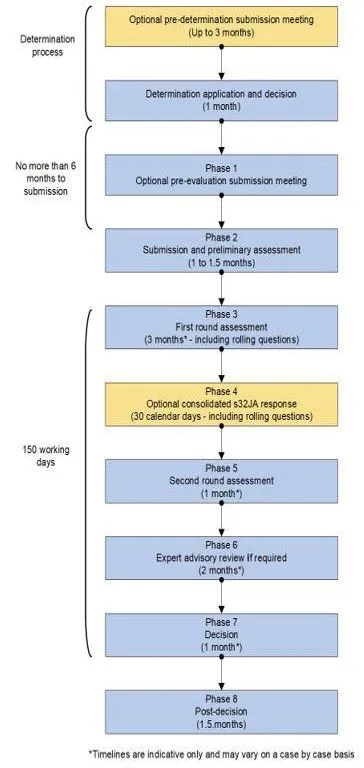
A flowchart showing a determination process with 9 phases.
The process begins with an optional pre-determination submission meeting (up to 3 months), followed by a determination application and decision (1 month).
Phase 1 shows an optional pre-evaluation submission meeting, leading to Phase 2's submission and preliminary assessment (1 to 1.5 months).
Phase 3 involves first round assessment (3 months, including rolling questions), followed by an optional consolidated s32JA response for Phase 4 (30 calendar days).
Phase 5 is second round assessment (1 month),
Phase 6 is expert advisory review if required (2 months), and Phase 7 is the decision (1 month).
The process ends with Phase 8, post-decision (1.5 months).
The flowchart indicates there should be no more than 6 months to submission, and notes a 150 working days timeframe.
A footnote states that timelines are indicative only and may vary on a case by case basis.
Eligibility for priority inclusion
Before you lodge your application for priority inclusion you need to:
- ensure that a priority determination is in force for the biological at the time of making a section 32DD application for inclusion. The application for inclusion of biological under section 32DD of the Therapeutic Goods Act 1989 is ‘made’ when it is submitted using the approved form or manner and is accompanied by specified information (the dossier)
- inform us of any material changes that may mean that the criteria for priority determination are no longer met, or that the section 32DD application will not be made while the determination is in force.
If you apply without a valid priority determination, your application will be treated as a standard application and will be processed according to the standard pathway processing timeframe.
Priority determination must be in force
A priority determination will cease to be in force 6 months from the date we notify you of our decision to make the priority determination unless:
- the determination is revoked
- a section 32DD application is made.
Priority determinations cannot be extended. If your priority determination is no longer in force, you must submit a new priority determination application and pay the applicable fee.
If you do not intend to re-apply for priority determination you may apply for inclusion via the standard biological inclusion pathway.
Application and evaluation fees
While application fees for priority inclusion and standard inclusion applications are the same, evaluation fees are higher for the priority inclusion. However, the processes for invoicing and payment of these fees are the same for both pathways.
The fee amounts (as set by Schedule 9 of the Therapeutic Goods Regulations 1990), are available at fees and payments.
Exit criteria from the priority pathway
Exit criteria have been established to define circumstances that may affect your priority application.
These exit criteria may be triggered at any time during the inclusion process. As a result, we may convert the application from the priority to the standard pathway (following assessment of the relevant circumstances).
The exit criteria are:
- failure to respond to our requests for additional information as part of a formal S32JA request within 30 calendar days
- identification of significant safety concerns that require further assessment (noting that the assessment of safety in the priority pathway will not be any less robust than for the standard pathway)
- submission of unsolicited or more extensive data than what is required during evaluation (excluding the provision of new safety related data, which you must bring to our attention)
- you are unlikely to meet the Good Manufacturing Practice (GMP) requirements for inclusion (that is, obtaining either an Australian manufacturing licence or GMP certification).
If the exit criteria are triggered and a decision is made to convert the application to the standard pathway, the priority review target timeframe of 150 working days will no longer apply. A mutual stop-clock will be applied and subsequent phases in the inclusion process adjusted. In these situations we will provide you with an updated evaluation plan.
Please note that no fees are refunded when a priority application is converted to a standard application due to triggering of exit criteria.
Pre-submission: phase 1
Sponsors with priority (applicant) determination can request a pre-submission meeting in the same way as standard applicants.
While optional, a pre-submission meeting is recommended for all priority applicants.
We recommend you arrange your pre-submission meeting as early as possible after receiving your determination notification. The determination is only valid for 6 months and you must lodge your priority inclusion application before it expires.
For further information refer to Pre-submission meetings with TGA.
Submission phase: phase 2
If there are any changes to the planned lodgement date of your application for inclusion (as advised in your determination application form), please email bloodandtissues@tga.gov.au as soon as possible.
This will assist us to allocate the required resources so that validation and evaluation processes can begin as soon as possible after we receive your submission.
Lodgement of your application for inclusion
The application submission phase is same as in standard biological inclusion pathway (see Applying for inclusion of a Class 2, 3 or 4 biological) except for the following:
- you must include a copy of the ‘priority determination notification letter’ in Module 1.5.2 of eCTD or provided along with your dossier at the time of submission.
- ensure that your submission supports the evidence that was presented in the determination application to demonstrate that you continue to meet the eligibility criteria for priority determination
- ensure that you have provided evidence of good manufacturing practice (GMP) for all manufacturing sites relevant to your priority submission. This includes either:
- existing approved Australian manufacturing licence or overseas GMP certification
- verification that you have applied to obtain either an Australian manufacturing license, overseas GMP certification, or GMP clearance as appropriate.
Evidence of GMP is a eligibility requirement for priority review. We will determine if you have met this requirement during the preliminary assessment of your submission.
We will evaluate your submission if it passes the preliminary assessment phase within the statutory timeframe of 30 working days (subregulation 16GC(2)) of the Therapeutic Goods Regulations 1990).
If your application passes preliminary assessment:
- our evaluation process starts and the priority evaluation will be completed within 150 days from the day we notify you and the clock starts (day 1)
- we send you an invoice for the evaluation fee within 7 days
- you have 28 days to pay your evaluation fee in full.
Priority evaluation fee
The fees for priority evaluations are higher than for standard evaluations.
An evaluation invoice is generated after we have completed our preliminary assessment and you have been notified of the outcome.
There are no provisions for payment by instalment and fees must be paid in full within 28 days via one of our payment options.
For the correct priority evaluation fees for your nominated class of biological refer to Schedule of fees and charges.
Evaluation plan
We will notify you of our acceptance of your submission for evaluation after it passes preliminary assessment. As with the standard pathway, notifications contain an evaluation plan with estimated dates.
We will only provide the start of phase 3 and end of phase 7 timepoints. Please note that unlike evaluations under the standard pathway, priority evaluations have no formal timelines between phase 3 and phase 7.
The end of phase 7 date in the evaluation plan assumes that there will be a 30 day stop-clock for responding to Section 32JA questions. This date may therefore change depending on the actual evaluation timeframes.
As evaluation plans are subject to change during the inclusion process you should always refer to our most recent correspondence for up-to-date timeframes.
Applications without priority determination
To use the priority review pathway all applications in your submission must have a priority determination.
Any related applications that do not have priority determination (either because they are ineligible or because you did not apply for determination) must be lodged separately via the standard biological inclusion pathway.
Note
If your submission includes both priority and standard applications, it will be considered to be a submission under the standard pathway and you will not be eligible for priority review.
Good Manufacturing Practice (GMP) requirements
At the time of lodging an application for priority inclusion you will need to:
- provide details of existing Australian manufacturing licences, overseas GMP certification
- provide evidence that you have applied to obtain either an Australian manufacturing license or GMP certification, as appropriate for all manufacturing sites.
It is your responsibility to follow up on lodged GMP applications and ensure all requirements are met before the inclusion decision (Phase 7).
If we determine that you are unlikely to meet GMP requirements for inclusion by phase 7 exit criteria will be triggered and your submission will be converted to the standard pathway.
For additional assistance refer to the Good Manufacturing Practice application decision tree.
First round of evaluation: phase 3
While this phase is the same as for the standard evaluation the timeframes are different.
The duration of this phase for priority applications is approximately 60 days. In comparison, the standard biological evaluation phase is allocated 100 days.
Rolling questions
You may receive questions (referred to as ‘rolling questions’) from us about your application at any time throughout the evaluation period
Given the nature of the evaluation process, it is not possible to predict in advance when questions will be asked during the first-round of evaluation.
We will continue to progress the evaluation while you prepare your responses to rolling questions. We will not link these rolling questions to a stop clock unless there are exceptional circumstances requiring us to do so.
Responding to rolling questions
You must provide your responses to rolling questions in a timely manner.
The usual timeframe for responses is 14 calendar days and will not exceed 30 calendar days. If you require additional time to provide a response, please email bloodandtissues@tga.gov.au.
You may provide us with a consolidated response to multiple requests for answers to rolling questions if the due dates are close.
Your responses to rolling questions must be provided as sequences of the submission dossier to bloodandtissues@tga.gov.au.
Evaluation reports
First round evaluation reports will not be provided at the end of the first round of assessment or with the consolidated section 32JA request (if required).
However, sufficient context will be provided with rolling questions and/or the consolidated section 32JA request for information to assist you in providing timely responses.
Consolidated section 32JA request response: phase 4
This phase is the same as for the standard biological inclusion pathway except for the following:
- any rolling questions identified 2 weeks prior to completion of phase 3 and/or any unanswered questions will be summarised into a consolidated section32JA (of the Therapeutic goods Act 1989) request and will result in a 30-day stop clock to allow you to prepare the response.
- if you do not respond within the 30-day stop clock your submission will be transitioned to the standard biological inclusion pathway as outlined in the priority exit criteria.
- if there are no outstanding questions at this stage, no section 32JA stop clock will occur and the submission will immediately proceed to the next phase (that is, both phases of evaluation will be combined, and the entire evaluation may be completed within 4 months).
Second round assessment: phase 5
This phase is the same as for the standard biological inclusion pathway.
When our evaluation is complete, you will receive the final evaluation report that includes consideration of your responses to the rolling questions and any section 32JA questions.
You will have at least two weeks after receipt of the final evaluation report to notify us of any errors of fact or major omissions.
If you choose to provide a response, this must be submitted as a validated sequence of the dossier.
Expert advisory review: phase 6
This phase is the same as for the standard biological inclusion pathway except for the following:
- the date on which your submission will reach this phase is subject to change as a result of the duration of the evaluation phase and whether a section 32JA stop clock is required. You will be provided with updates to your evaluation plan during the inclusion process as required.
- the priority pathway allows for flexibility in the expert advisory review phase. All submissions are initially scheduled to be considered by the Advisory Committee on Biologicals (ACB). However, committee advice will not always be required. To meet timeframes for the priority review process, submissions may be considered either at a scheduled committee meeting or out of session.
- we may also seek expert advice other than through the ACB.
The timeframes and procedures for exchange of information between you and the TGA during this phase are the same as for the standard biological inclusion pathway.
As per the standard pathway, you will also receive a copy of the TGA delegate’s overview of your submission.
Decision: phase 7
This phase is the same as for the standard biological inclusion pathway.
Post-decision: phase 8
This phase is the same as for the standard biological inclusion process.
Page history
Amended related links. removed the prescription medicines link and added biologicals (priority applicant) determination and form.
Original publication.
Amended related links. removed the prescription medicines link and added biologicals (priority applicant) determination and form.
Original publication.

