Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is for sponsors and manufacturers supplying medicines in Australia that are serialised or have data matrix codes. It describes the requirements in the Therapeutic Goods (Medicines – Standard for Serialisation and Data Matrix Codes) (TGO 106) Order 2021.
To understand the meaning of terms used in this guidance refer to:
- machine-readable codes
- serialised medicines
- relevant level of packaging.
Legislation
About TGO 106
The purpose of TGO 106 is to provide clarity for adopters of serialisation and data matrix codes on medicines supplied in Australia. It is the first step in establishing requirements that support all systems relying on the codes.
TGO 106 sets out the requirements for medicines supplied in Australia where:
- a medicine is serialised
- a data matrix code containing a Global Trade Item Number (GTIN) is applied to a medicine.
Where possible TGO 106 requirements align with global standards to provide consistency for sponsors and manufacturers operating in multiple jurisdictions and to ensure global interoperability.
TGO 106 does not set out any requirements regarding reporting, storage and verification of serialisation data.
TGO 106 is a standard made under section 10 of the Therapeutic Goods Act 1989.
Commencement date
Medicines subject to TGO 106 that are released for supply from 1 January 2023 must comply with TGO 106.
Medicines that are subject to TGO 106 requirements
Medicines supplied in Australia are subject to TGO 106 requirements if they:
- are serialised
- include a data matrix code which encodes the GTIN.
Refer to medicines that are exempt from TGO 106 for information on medicines that do not need to comply with TGO 106.
The flowchart below illustrates when the requirements of TGO 106 apply and when they do not.
When requirements of TGO 106 apply
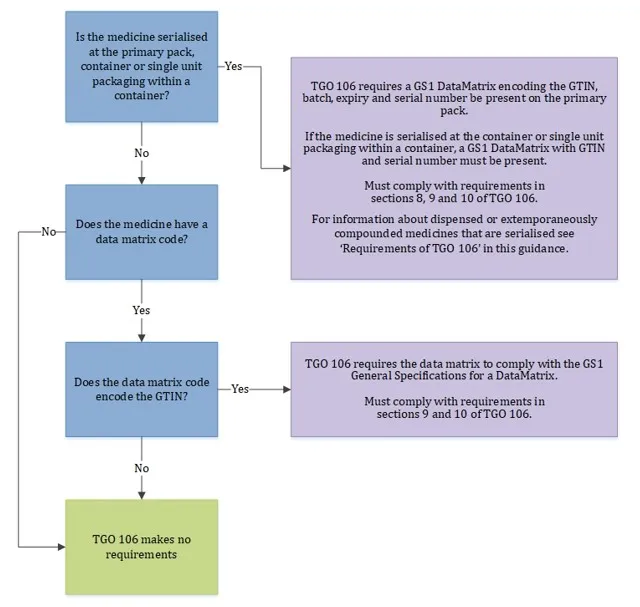
Flowchart showing when TGO 106 requirements apply to medicine packaging.
The chart has 3 decision points and 3 possible outcomes.
- Starting point: 'Is the medicine serialised at the primary pack, container or single unit packaging within a container?'
- If YES: Leads to first outcome: TGO 106 requires a GS1 data matrix encoding the GTIN, batch, expiry and serial number be present on the primary pack. If the medicine is serialised at the container or single unit packaging within a container, a GS1 data matrix with GTIN and serial number must be present. Must comply with requirements in sections 8, 9 and 10 of TGO 106. Additional note: For information about dispensed or extemporaneously compounded medicines that are serialised see 'Requirements of TGO 106' in this guidance.
- If NO: Leads to second question: 'Does the medicine have a data matrix code?'
- If NO to second question: Leads to second outcome: 'TGO 106 makes no requirements'
- If YES to second question: Leads to third question: 'Does the data matrix code encode the GTIN?'
- If YES to third question: Leads to third outcome: TGO 106 requires the data matrix to comply with the GS1 General Specifications for a data matrix. Must comply with requirements in sections 9 and 10 of TGO 106.
- If NO to third question: Leads back to 'TGO 106 makes no requirements'.
The flowchart uses colour coding with blue for decision points, purple for requirement outcomes, and green for the 'no requirements' outcome.
Data matrix codes that do not encode the medicine’s GTIN (and where the medicine is not serialised) have no further requirements. That is, they can be in any format and a human-readable transcription is not required.
See also: Example primary pack that is compliant with TGO 106.
Medicines that are exempt from TGO 106
Section 6 of TGO 106 identifies medicines that are not required to comply with TGO 106 and these are described below.
TGO 106 does not preclude these medicines from complying if the medicine manufacturer or sponsor chooses to do so.
Export only medicines
Medicines that are manufactured in Australia for export or imported into Australia for the purposes of export as per Section 3 of the Therapeutic Goods Act 1989.
Blood or blood product
Medicines that are funded under the national blood arrangements are required to bear a GS1 data matrix or ISBT 128 barcode. Where the barcode specifications are met, TGO 106 is considered to be complied with.
If the National Blood Authority ceases to prescribe barcoding requirements, these medicines must then comply with TGO 106.
Medicines supplied under special circumstances
Medicines supplied with an approval under Section 19 or 19A of the Therapeutic Goods Act 1989 include medicines for clinical trials, those supplied under the Special Access Scheme, or are supplied due to unavailability or shortage of registered medicines.
GS1 provides a standard for the identification of investigational products in healthcare clinical trial processes.
Medicines imported for use by immediate family
Medicines mentioned in Item 1 of Schedule 5 to the Therapeutic Goods Regulations 1990 refers to therapeutic goods that are imported for use in the treatment of the importer or the importer’s immediate family in certain circumstances.
Machine-readable codes
A machine-readable code is data encoded into a format that can be read by an electronic device.
Examples include linear barcodes, 2D-barcodes (such as QR codes, Aztec and data matrix codes) and radio-frequency identification (RFID) tags.
Examples of machine-readable codes

A horizontal comparison of 5 different types of machine-readable codes, arranged from left to right:
- linear barcode (traditional black and white vertical lines with numbers below)
- data matrix (a square black and white pattern)
- QR code (a square black and white pattern, larger than the data matrix)
- Aztec Code (a square black and white pattern with a distinct target-like centre)
- RFID tag (shown as a copper-coloured square spiral antenna pattern).
Each code type is labelled below with its name. These examples represent different methods for storing and transmitting machine-readable data.
Data matrix code
A data matrix code is a type of two-dimensional code that can be read by a scanner. It is a small square or rectangle with two solid edges, two dotted edges and pixelated light and dark areas within the matrix. There are no fixed shapes within the matrix.
For the purposes of TGO 106 and this guidance, QR codes are not considered data matrix codes. QR codes are another type of two-dimensional code with some similarities in appearance to data matrix codes. However, unlike data matrix codes, QR codes have large squares in the corners of the code.
DataMatrix
A DataMatrix is data matrix code formatted in accordance with the Global Standards 1 (GS1) General Specifications.
Below is an example of a DataMatrix encoding 4 data elements - GTIN, batch number, expiry date and serial number:
Example DataMatrix encoding 4 data elements

A DataMatrix barcode containing tracking information with the following text displayed below it:
- GTIN: 012345678900000
- LOT: XC2312
- EXP: 31/01/2020
- S/N: 555643225C.
Serialised medicines
A serialised medicine is one where each unit bears a unique identifier, allowing the unit to be identified distinctly within its batch. This typically is achieved through a serial number applied to the unit. The combination of product number (GTIN) and serial number creates a globally unique character chain for the unit.
A definition of serialisation is provided in Section 4 of TGO 106.
Relevant level of packaging
Relevant level of packaging is a term included in TGO 106 to explain that the requirements relate to the level at which a medicine is serialised or has a data matrix code. If, for example, the medicine is serialised at the primary pack, the requirements relate to the primary pack. It would not relate to packaging contained within the primary pack.
There are differences in how Australia refers to packaging compared to other standards about packaging levels. Details and examples of packaging terminology can be found at Medicine packaging definitions for sponsors.
Primary pack
Primary pack has the same meaning as in the Therapeutic Goods Act 1989 where it is defined as ‘the complete pack in which the goods, or the goods and their container, are to be supplied to consumers.’
Primary pack is different to primary packaging.
The primary pack may be what others refer to as ‘primary packaging’ or ‘secondary packaging’, depending on the product.
The primary pack as defined in the Act is usually secondary packaging in GS1 and Good Manufacturing Practice (GMP) guidance.
Sometimes the primary pack is also primary packaging, such as a bottle of capsules with no further packaging.
Example primary pack that is compliant with TGO 106
The example primary pack label below contains a linear barcode encoding the GTIN and a data matrix code on a flap. The data matrix encodes a reference number for the label which is common to all labels for the product and is not unique to the pack. It does not encode the medicine’s GTIN.
This pack is not serialised, and the data matrix does not encode the GTIN, therefore no changes to the packaging are required under TGO 106.
Example of a primary pack
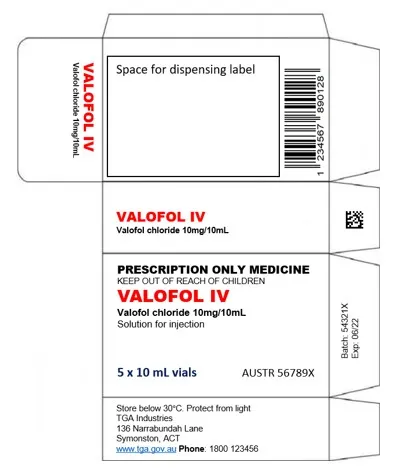
Medication box layout for Valofol IV (valofol chloride 10mg/10mL).
The unfolded box template shows multiple panels including:
- a space for dispensing label
- product name
- prescription-only warning
- dosage information stating '5 x 10 mL vials'
- storage instructions 'Store below 30°C'
- a barcode
- manufacturer details including an Australian address and phone number.
The text 'PRESCRIPTION ONLY MEDICINE' and 'KEEP OUT OF REACH OF CHILDREN' are prominently displayed. A DataMatrix code is visible on one panel.
Primary packaging
Primary packaging, as used in GS1 and GMP guidance, is the packaging which directly contacts the medicine (injection vial, tablet blister and so forth). The Therapeutic Goods Act 1989 refers to this as the container.
Container
Container has the same meaning as in the Therapeutic Goods Act 1989 where it is defined as ‘the vessel, bottle, tube, ampoule, syringe, vial, sachet, strip pack, blister pack, wrapper, cover or other similar article that immediately covers the goods, but does not include an article intended for ingestion.’
Single unit packaging within a container
An example of single unit packaging within a container is the segment of a blister pack containing a capsule where it can be readily detached.
Requirements of TGO 106
Part 2 of TGO 106 outlines the requirements for medicines subject to TGO 106.
Medicines that are serialised are subject to the requirements outlined in Section 8, 9 and 10 of TGO 106.
Medicines that are not serialised but have a data matrix code that contains a GTIN must comply with Sections 9 and 10.
Section 8 and 10 include specific requirements for the primary pack. Primary pack has a different meaning to primary packaging.
Dispensed or extemporaneously compounded medicines as outlined in subsection 7(2) and 7(3) that are serialised using a method other than the application of data matrix codes are not subject to the requirements in Section 8. If these medicines have a data matrix code with a GTIN for another purpose (that is, the code does not contain the serial number) then they are subject to the requirements in Sections 9 and 10. The intention is that if these medicines are serialised then you do not have to add a data matrix code. However, application of data matrix codes as outlined in TGO 106 is the recommended method for serialising medicines.
Serialised medicines and application of data matrix codes
Where a medicine is serialised this must be represented by a serial number encoded into a data matrix code that complies with the GS1 General Specifications for a DataMatrix. No other method of serialising complies with the standard. Batches cannot be partially serialised.
The GTIN and serial number should combine to afford a globally unique number for the unit. GS1 General Specifications state that serial numbers should not be duplicated for a GTIN.
Where a medicine has a data matrix code that contains a GTIN, but is not serialised, it must also comply with the GS1 General Specifications for a DataMatrix. See also: Formatting of data matrix codes.
See also Requirements of TGO 106 for information about dispensed or extemporaneously compounded medicines that are serialised.
Serial numbers
GS1 General Specifications limit the serial number to 20 alphanumeric characters.
Serial numbers are recommended to be randomised.
All alphanumeric characters allowed for use in serial numbers as listed in the GS1 General Specifications are acceptable. However, we recommend that serial numbers contain only numerical digits.
To assist with human-readability of transcribed information we recommend that if using characters that are not numbers, to:
- exclude letters that may cause confusion with similarly shaped characters, for example o, and O
- avoid mixtures of upper- and lower-case letters
- avoid symbols if possible.
Data elements
The minimum data elements required to be encoded in the DataMatrix under TGO 106 vary depending if the medicine is serialised and on the relevant level of packaging.
Section 8 of TGO 106 outlines the data elements required in a data matrix code when a medicine is serialised.
If a medicine primary pack is serialised, a DataMatrix must be applied with the following data elements:
- GTIN
- batch number
- expiry date
- serial number.
See also data matrix code on a primary pack.
If a medicine container or single unit packaging within a container is serialised, the following data elements must be encoded in the data matrix code:
- GTIN
- serial number.
If a medicine is not serialised, there are no requirements for additional data elements to be included within the DataMatrix.
Other data elements can be included subject to GS1 General Specifications and TGO 106.
| Action | Minimum data elements required (and GS1 Application Identifier) | Where in TGO 106 |
|---|---|---|
| Serialise medicine at primary pack | GTIN (01) Batch number (10) Expiry date (17) Serial number (21) | Section 8 |
| Serialise medicine at container or single unit packaging within a container | GTIN (01) Serial number (21) | Section 8 |
| Apply a data matrix code with a GTIN to a medicine (medicine is not serialised) | GTIN (01) | No additional data elements required under TGO 106 |
GS1 Application Identifiers
GS1 Application Identifiers (AIs) are prefixes used to identify data in barcodes as outlined in the GS1 General Specifications.
Formatting and durability of matrix codes
If a data matrix code containing a GTIN is applied to a unit of a medicine, this must be formatted as a GS1 DataMatrix that complies with the GS1 General Specifications.
Section 9 of TGO 106 also outlines requirements for the placement of data matrix codes. See: Multiple machine-readable codes.
Durability of data matrix codes
A DataMatrix must be machine-readable for the life of the product.
Where a code is smudged, faded or otherwise unreadable, the medicine is not compliant with TGO 106.
We recommend you consider measuring machine-readability in your medicines stability studies in the same way as batch and expiry printing.
Information in a data matrix code
The information in the DataMatrix must agree with all machine-readable and human-readable information on the label as well as any relevant content of the Product Information (PI) or Consumer Medicine Information (CMI). If for example the expiry date encoded in the DataMatrix does not agree with the human-readable expiry on the label, the medicine is not compliant with TGO 106. See also: GTINs in multiple machine-readable codes.
A DataMatrix may be formatted to enable retrieval of data from a remote location. This may allow other material such as the medicine’s PI or CMI to be accessed via a remote database. Where this capability is included in the DataMatrix, the documents accessed must be the current and/or approved versions of those documents.
Any information must be linked to or provided in accordance with TGA legislation including the Therapeutic Goods Act 1989 and the Therapeutic Goods Advertising Code.
Human readability
Information contained in a DataMatrix should be transcribed on the label in human-readable format as prescribed by the GS1General Specifications.
There may be other labelling requirements that apply to some human readable information for example, where medicines must comply with TGO 91 or TGO 92.
Ordering of human-readable data elements
The human-readable data element fields do not have to be presented in any specific order.
Human-readable prefixes or headers do not have to be placed in any specific location. However, when applied to a primary pack they must allow the data to be interpreted without knowledge of the GS1 General Specifications. See: Human-readable information on primary packs.
Human-readable information on primary packs
TGO 106 mandates how human-readable information is to be applied to a primary pack.
Human-readable information must be located adjacent to the DataMatrix, where space permits, in accordance with GS1 General Specifications.
In addition to the GS1 General Specifications, when a DataMatrix is applied to a primary pack, the human-readable information must be presented such that no knowledge of GS1 formatting is required for the information to be understood.
The DataMatrix example below is not compliant with human-readability requirements. While the human-readable data accurately reflects the encoded data, which includes the GTIN, expiry, batch and serial number, it is essentially meaningless to anyone unfamiliar with GS1 DataMatrix formatting.
Human-readable example

A data matrix barcode containing encoded information without human-readable text labels. The barcode appears in black against a white background in a rectangular format.
Shown below is the same data matrix amended to be compliant with human-readability requirements.
The human-readable information is presented to allow the data to be readily understood.
Parenthesised prefixes have been replaced with abbreviations.
Each field is on a separate line and the expiry date has been formatted for easy interpretation.
DataMatrix that is compliant with human-readability requirements

A Data Matrix barcode with accompanying human-readable text labels below it. The barcode is black against a white background, and includes clearly visible text showing: GTIN: 012345678900000; LOT: XC2312; EXP: 31/01/2020 S/N: 555643225C
Medicines subject to TGO 91 or TGO 92
The GS1 General Specifications set out human readable interpretation rules including requirements for font and text size. Medicine labelling requirements may also apply to some of this information as outlined in Therapeutic Goods Order No. 91 – Standard for labels of prescription and related medicines (TGO 91) and Therapeutic Goods Order No. 92 – Standard for labels of non-prescription medicines (TGO 92) and associated guidance.
If for example the expiry date in the human-readable interpretation of the data matrix code is the only version of the expiry date on the medicine label, this will need to comply with the requirements for text size and prefixes set out in TGO 91/92.
TGO 91 and TGO 92 always requires the medicine expiry to be present on the container.
Multiple machine-readable codes
Medicines may include more than one machine-readable code on their packaging for a variety of reasons including:
- transitioning to a GS1 DataMatrix but still requiring linear EAN barcodes to support existing technology
- regulatory requirement for a code from the country of manufacture
- the label contains a QR code for consumer or healthcare practitioner reference.
GTINs in multiple machine-readable codes
Where multiple machine-readable codes containing a GTIN are printed on a medicine package, all codes must encode the same GTIN.
If a linear barcode (EAN-13) is on a pack containing a GTIN-13 identification number, the GTIN-14 for the GS1 DataMatrix is created by adding a leading 0 (zero) to the GTIN-13 in the original barcode to ensure that the number remains the same.
Placement of machine-readable codes on packaging
To minimise the risk of reading the wrong code, machine-readable codes should be physically distanced from each other.
In the case of primary packs, where possible print the DataMatrix on a side of the pack devoid of other machine-readable codes.
On round surfaces, where possible separate the DataMatrix by at least 60 degrees from any other machine-readable code.
The label below shows a label with multiple codes. As the QR code on the label does not contain a unique identifier for the pack, no further action is required.
Example label with multiple codes that does not require a DataMatrix

Medication label for Flying Fish Brand Vitamin C Tablets.
Contains 100 tablets of 100mg Ascorbic acid per tablet. Manufacturer: TG Industries.
Storage instructions: Store below 30°C.
Contact details: 124 Narrabundah Lane, Symonston ACT,
website: tga.gov.au, phone: 1800 123 456.
Registration number: AUST L 58836X.
Lot number: 7007145, Expiry: 09/2020.
Label includes a blue flying fish logo and QR code.
Where a QR code contains a serial number or other method of uniquely identifying the unit within the batch, a DataMatrix is required as shown on the label below.
Example label with multiple codes that requires a DataMatrix

Medication label for Flying Fish Brand Vitamin C Tablets, showing an updated design.
Contains 100 tablets of 100mg Ascorbic acid per tablet. Manufacturer: TG Industries.
Storage instructions: Take tablets as required with water, store below 30°C.
Contact details: 124 Narrabundah Lane, Symonston ACT, website: tga.gov.au, phone: 1800 123 456.
Registration number: AUST L 58836X.
Additional unique identifier code present below main QR code. Label includes a blue flying fish logo.
The DataMatrix contains the GTIN, batch, expiry and serial number. The data in the DataMatrix must also match the other machine-readable codes on the label. The 13-digit GTIN in the linear code has been converted to a 14-digit code for the DataMatrix.
As the label is for a round bottle, the DataMatrix and QR codes are separated by 60 degrees to reduce the risk of an unintentional scan.
Linear barcodes
Sponsors are encouraged to include linear barcodes on medicines if transitioning to data matrix codes to support existing technology and scanning by healthcare professionals as part of patient safety requirements. There are no specific requirements outlined in TGO 106 regarding linear code replacement.
Linear barcodes on medicines are widely scanned in Australia to provide many benefits including efficiencies at point of sale, while their scanning in clinical settings may reduce medication errors.
QR Codes
QR codes are permitted to be present on the same labelling as a data matrix code and may contain the same information as the data matrix code, some of same information, or none of it.
If the QR code contains a number or link that is unique to the unit of medicine it is printed on, the unit is considered to be serialised and a DataMatrix must be printed containing the serial number as this is a medicine that is subject to TGO 106 requirements. The information in the DataMatrix must agree with all machine-readable information on the label. See: Information in a data matrix code.


Data matrix code on a primary pack
A data matrix code on a primary pack label that encodes a GTIN must be formatted in accordance with the GS1 General Specifications for a DataMatrix. This ensures a single, globally recognised format for the data encoded in the matrix.
If the medicine is not serialised, there are no requirements for additional data elements to be included within the DataMatrix. In accordance with the GS1 General Specifications however, other information such as the batch, expiry and manufacturing date, may be included at the sponsor’s discretion.
Serialised primary pack
If a medicine primary pack is serialised, a DataMatrix must be applied with the following data elements:
- GTIN
- batch number
- expiry date
- serial number.
In accordance with the GS1 General Specifications, other information such as manufacturing date, may be included at the sponsor’s discretion.
See also: data elements.
Example serialised medicine primary pack label that is compliant with TGO 106
The DataMatrix on the primary pack below is formatted to GS1 General Specifications.
- It contains the mandatory elements of GTIN, batch, expiry and serial number.
- The information within the DataMatrix is printed adjacent to the code with prefixes to enable a user to interpret the information.
- The DataMatrix is printed on a face without any other machine-readable codes.
- The data matrix code on the flap does not contain the GTIN so may remain without any changes.
An example of a serialised primary pack

Unfolded pharmaceutical box template for VALOFOL IV (Valofol chloride 10mg/10mL solution for injection).
The template shows various panels of the box including:
- Product name "VALOFOL IV" in red text
- "PRESCRIPTION ONLY MEDICINE" warning
- "KEEP OUT OF REACH OF CHILDREN" warning
- Dosage strength: 10mg/10mL
- Pack size: 5 x 10 mL vials
- Registration number: AUSTR 56789X
- Sponsor address
- Contact phone number
- Manufacturer's website
- Space marked for dispensing label
- Multiple barcodes and data matrix codes
- Folding guide lines.
The template appears to be a 2D production artwork layout showing how the box will be printed and folded.
Labelling of logistic units
Where serialised units are packed into bundles, shippers, pallets etc. it is recommended these logistic units be labelled so as to allow the creation of a hierarchical, parent-child relationship between the packaging levels.
Each packaging level should be uniquely identified using global standards. This will enable accurate identification of medicines across the multiple levels of the hierarchy – from the container (and in some cases single unit packaging within a container) to primary pack through to units managed in transport or logistics.
A discussion paper providing guidance on the implementation of aggregation in the pharmaceutical supply chain using GS1 standards is available on the GS1 website.

