Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is intended for consumers, health professionals and industry. It provides a general overview of the Australian legislative framework for low risk (listed) medicines and registered complementary medicines. It explains the different types of medicines and the different entry pathways into the Australian Register of Therapeutic Goods (ARTG). It also provides information on legislation, medicine ingredients, medicine interface issues, practitioner and product exemptions, supplying medicines and post market monitoring.
For information
For information:
For all guidance and resources relevant to listed medicines and registered complementary medicines refer to Australian Regulatory Guidelines for Listed Medicines and Australian Regulatory Guidelines Registered Complementary Medicines.
Overview of the regulation of medicines in Australia
The regulatory framework for therapeutic goods regulates products according to risk. Our scientific assessments and decision-making are intended to ensure that the benefits of a medicine outweigh any risks associated with its use.
For the TGA to maintain public confidence in the safety, benefits and risk management associated with medicines supplied onto the Australian market, the TGA generally regulates medicines in two ways:
- pre-market, before a medicine is permitted be supplied
- post-market, after a medicine has been supplied.
For more information about supplying medicines in Australia, see Supply a therapeutic good.
Australian Register of Therapeutic Goods (ARTG)
Unless exempt (refer to Medicines exempt from certain TGA regulation) medicines must be entered in the Australian Register of Therapeutic Goods (ARTG) before they can be legally imported, exported, manufactured or supplied for use in Australia. Each medicine included in the ARTG has a unique ARTG identification which starts with 'AUST', followed by 'L', 'L(A)' or ‘R’ with numbers (see Classification of medicines as registered or listed). This ARTG number is displayed on the front label of the medicine.
The regulatory requirements, levels of assessment, timeframes and fees to enter a product in the ARTG increase with an increasing degree of risk. For more information see Entering medicines in the ARTG.
Classification of medicines as registered or listed
Australia has a tiered system for regulating medicines, based on risk. Medicines are classified as below:
- Lower risk medicines are listed in the ARTG, there are two types of listed medicines:
- Listed medicines are the lowest risk medicines as they can only use low-risk ingredients and low-level indications (health claims) that must be selected from TGA approved lists (the Permissible Ingredients and Permissible Indications Determinations). Listed medicines are not individually evaluated by the TGA before they are released onto the market; instead, they are automatically included in the ARTG following completion of an application and certification by the sponsor that their product meets all applicable legislative requirements in relation to safety, quality and efficacy. These medicines have an AUST L number on their medicine label. Most listed medicines in the ARTG are complementary medicines but some over-the-counter (OTC) products such as sunscreens and dental products are also listed in the ARTG.
- Assessed listed medicines are slightly higher risk than listed medicines. Like other listed medicines, assessed listed medicines can only contain pre-approved low risk ingredients and are entered in the ARTG following self-certification by the applicant of the safety and quality of the product. These medicines differ from other listed medicines in that they can make higher level ‘intermediate’ indications and because of this, they undergo a TGA pre-market assessment of the evidence supporting the product’s indications. These medicines have an AUST L(A) number on their medicine label.
- Higher risk medicines are registered in the ARTG. Registered medicines have a higher level of risk due to their ingredients (e.g. ingredients included in a Schedule to the Australian Poisons Standard) or therapeutic indications (e.g. referring to the treatment of serious conditions) and are required to be ‘registered’ in the ARTG. These medicines undergo a full TGA evaluation (‘approval’) for safety, quality, and efficacy. Medicines that are required to be registered in the ARTG include prescription medicines (e.g. antibiotics), over-the-counter medicines (e.g. paracetamol) and registered complementary medicines (e.g. high dose iron supplements). These medicines have an AUST R number on their label. A small number of complementary medicines are registered in the ARTG.
See Diagram 1 for different medicine types that are regulated as listed or registered medicines. More information on the classification of medicines is provided at Entering medicines in the ARTG.
Diagram 1: Different medicine types regulated as listed or registered medicines
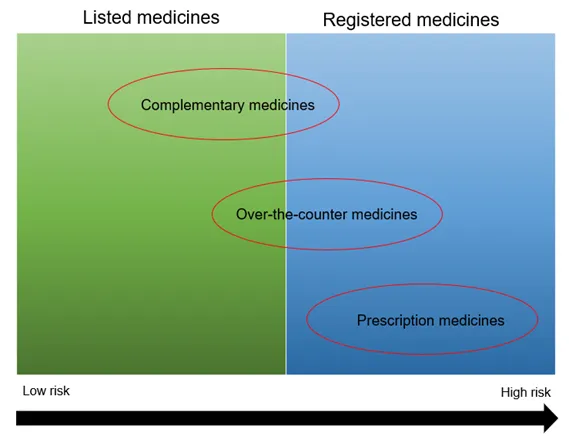
- The image is divided into two columns, the left column is listed medicines, and the right column is registered medicines.
- At the bottom is an arrow running from left to right denoting low risk to the left and high risk to the right.
- The oval shape containing words ‘Complementary medicines’ is predominantly in the listed medicines column with a small portion in the registered medicine column.
- The oval shape containing the words ‘over-the-counter medicines’ is predominantly in the registered medicines column with a small portion in the listed medicine column.
- The oval shape containing the words ‘prescription medicines’ is only in the registered medicines column.
Legislation and guidance
Legislation and guidance
All products within the scope of the framework need to comply with the requirements set out in the relevant legislation administered by the TGA.
Important:
Legislation is amended from time to time, as such, it is important that sponsors (product owners) refer to the current ‘in-force’ version of legislation, which can be accessed from the Federal Register of Legislation.
The legislative basis for a uniform national framework of controls for the import, export, manufacture and supply of complementary medicines are:
- the Therapeutic Goods Act 1989 (the Act)
- the Therapeutic Goods Regulations 1990 (the Regulations)
There are also various legislative instruments, Standards and Orders, which provide further details about the matters covered by the Act, refer to the following TGA webpages:
Standards
All medicines and ingredients used in the manufacture of therapeutic goods must comply with applicable standards, in most cases, these are ‘default standards’, which are publicly available authoritative standards provided by the British Pharmacopoeia, the European Pharmacopoeia and the United States Pharmacopeia – National Formulary. A default standard is defined in the Act as statements in monographs (except for statements or monographs exempted by the Minister), as interpreted in accordance with the General Notices section of the relevant pharmacopoeia.
In addition, the minister may, by legislative instrument (under section 10 of the Act), make a ‘ministerial standard’, that specifies alternative or additional requirements to a default standard. A ministerial standard is also known as a Therapeutic Goods Order (TGO). A ministerial standard may specify requirements where there is no default standard applicable to a therapeutic good or may specify additional requirements to those already specified in a default standard. In some cases, a ministerial standard may specify requirements that are different to a default standard. The ‘Application’ section at the start of a TGO determines whether a TGO is applicable to the goods.
Important:
Any matter specified in a ministerial standard (TGO) (under section 10 of the Act) has precedence over requirements of a default standard where the default standard is inconsistent with the relevant TGO.
Refer to Complying with ministerial and default standards for medicines for further information about default and ministerial standards.
Other standards, such as Australian/New Zealand Standards, can be adopted by the TGA (refer to Part 3-1 paragraph 10(2)(a) of the Act) by including a reference to the document in therapeutic goods legislation.
For information:
Consent to supply goods not compliant with applicable standards
Therapeutic goods must comply with Part 3-1 (Standards) of the Act. However, a sponsor can apply, under sections 14 and 14A of the Act, to request consent to the import, supply or export of goods that do not comply with applicable standards (or aspects of an applicable standard). Such requests incur an application fee. For more information refer to:
Other legislation and requirements
Sponsors and businesses involved in transport and supply of materials and the supply of consumer goods should be aware of other Australian legislation and requirements which might be applicable to their medicine, such as:
- Environment Protection and Biodiversity Conservation Act 1999
- Food Standards Australia New Zealand Act 1991
- Customs Act 1901 and the Customs (Prohibited imports) Regulations 1956
- Industrial Chemicals Act 2019 and Industrial Chemicals (General) Rules 2019
- Gene Technology Act 2000 and the Gene Technology Regulations 2001
- Competition and Consumer Act 2010 and the Australian Consumer Law
- National Measurement Act 1960
- Australian Dangerous Goods Code
- Agricultural and Veterinary Chemicals Act 1994.
Sponsors should also be aware of requirements that may be applicable under other Australian State and Territory legislation.
Guidance and resources for listed medicines and registered complementary medicines
Refer to Australian Regulatory Guidelines for Listed Medicines and the Australian Guidelines for Registered Complementary Medicines for a list of guidance and resources applicable to listed medicines and registered complementary medicines.
What are complementary medicines?
Part 1(2) of the Regulations provides the following definitions:
Complementary medicine means a therapeutic good consisting wholly or principally of 1 or more designated active ingredients, each of which has a clearly established identity and a traditional use.
Designated active ingredients, for a complementary medicine, means an active ingredient, or a kind of active ingredient, mentioned in Schedule 14 (to the Regulations):
Designated active ingredients
- an amino acid
- charcoal
- a choline salt
- an essential oil
- plant or herbal material (or a synthetically produced substitute for material of that kind), including plant fibres, enzymes, algae, fungi, cellulose and derivatives of cellulose and chlorophyll
- a homoeopathic preparation
- a microorganism, whole or extracted, except a vaccine
- a mineral including a mineral salt and a naturally occurring mineral
- a mucopolysaccharide
- non-human animal material (or a synthetically produced substitute for material of that kind) including dried material, bone and cartilage, fats and oils and other extracts or concentrates
- a lipid, including an essential fatty acid or phospholipid
- a substance produced by or obtained from bees, including royal jelly, bee pollen and propolis
- a sugar, polysaccharide or carbohydrate
- a vitamin or provitamin
Table 1 provides some examples of products that are regulated as complementary medicines.
| Medicine type | Description |
|---|---|
| Traditional medicines | Traditional medicines include a diverse range of health practices, approaches, knowledge and beliefs incorporating medicines of plant (including algal, yeast or fungal), animal and/or mineral origin. Examples of traditional paradigms include: Traditional Chinese Medicine (TCM), Ayurvedic medicine; Australian indigenous medicine, Western herbal medicine. ‘Traditional use’ is defined in Part 1(2) of the Regulations: for a designated active ingredient, means use of the designated active ingredient that:
In general, an established tradition of use is considered to exceed three generations of human use, equating to approximately 75 years. |
| Herbal medicines | Herbal medicines are therapeutic goods that are or contain herbal substances as the major active ingredient(s). Herbal substances are defined in Part 1(2) of the Regulations: herbal substance means all or part of a plant or substance (other than a pure chemical or a substance of bacterial origin):
Herbal substances include preparations of plants and other organisms that are treated as plants in the International Code of Botanical Nomenclature, such as fungi, algae and yeast. The current regulatory system provides for listed medicines to contain a wide range of herbal substances provided that it can be adequately demonstrated that the herbal substance is safe. |
| Homoeopathic medicines | As defined in the Act, a ‘homoeopathic preparation’ is a preparation:
mother substance means any of the following:
Under the Regulations, a homoeopathic preparation means a preparation:
Homoeopathic medicines are based upon the central tenet of homoeopathy ‘let like cure like’ and the principles of homoeopathic pharmacy - ‘potentisation’, the serial dilution and succussion of a mother tincture. The term ‘mother tincture’ means a preparation by the process of solution, extraction or trituration to prepare homoeopathic preparations. A mother tincture is the first preparation of the mother substance and provides the base for further homoeopathic dilutions. Homoeopathic medicines are manufactured to different medicinal strengths or ‘potencies’ according to manufacturing standards described in homoeopathic pharmacopoeias. Homoeopathic medicines are derived from a wide variety of natural source materials, mostly plants and minerals. Some of these source materials are poisonous, for example: Atropa belladonna. The highly diluted nature of homoeopathic preparations is considered to render starting materials non-toxic and therefore safe for therapeutic use. For more information on the regulation of homoeopathic medicines refer to Homoeopathic preparations included/exempt from inclusion in the ARTG. |
| Anthroposophic medicines | As defined in the Act, an anthroposophic preparation is a preparation:
Anthroposophic practitioners use a range of interventions including conventional therapies, remedies based upon homoeopathic principles, herbal medicine and external therapies. |
| Essential oils | Essential oils are highly concentrated oils. The TGA’s Plant Preparation Code Table defines an essential oil plant preparation as: "An oil that is largely or completely composed of volatile oils, and is usually obtained via one or more distillations with steam, water alone, or water with alcohol. The essential oils of Citrus peels are obtained by pressing. Citrus juice 'essence' oils are distilled. Essential oil 'absolutes' are prepared by extraction in a non-polar solvent and a second extraction in alcohol. Volatile herbal oils are complex mixtures of hydrocarbons and oxidation products of hydrocarbons; they gradually evaporate at room temperature and are often fragrant." The purpose of a product containing an essential oil determines which agency regulates it for example, if the product makes only cosmetic claims it is likely to be considered a cosmetic and regulated by the Australian Industrial Chemicals Introduction Scheme (AICIS), but if the product makes a therapeutic claim it could be considered a therapeutic good and be regulated by the TGA. Sponsors of products containing essential oil(s), which are considered to be therapeutic goods must comply with all statutory requirements, including: the default standards, the Poisons Standard, Therapeutic Goods labelling Order as current and in force and Therapeutic Goods Order No. 95 – Child-Resistant packaging requirements for medicines 2017 (TGO 95). Essential oils that are supplied solely as starting materials to practitioners are generally exempt from the requirement to be included in the ARTG before supply. |
| Nutritional supplements | Nutritional products used to supplement the diet may be regulated as foods or therapeutic goods depending upon such things as their ingredients, health claims and presentation - refer to Medicine interface issues. Examples of nutritional substances that are considered to be complementary medicines, and usually regulated as listed medicines, include vitamin and mineral supplements. Many vitamins and minerals are included in a Schedule to the Poisons Standard and in accordance with this scheduling, such things as pack size and container dimensions may be limited. Other nutritional substances that may be therapeutic goods, if presented as such, include fish oils, shark cartilage, krill oil, amino acids. |
Other types of complementary medicines such as flower, shell and gem essences (which are highly diluted extracts of substances) are generally not regulated as medicines in Australia, unless they have therapeutic indications.
Medicine interface issues
There can be a regulatory 'interface', or potential overlap, between certain foods, medicines, devices and cosmetics. For example, even though a product may meet the definition of a therapeutic good, it may:
- be regulated as a different type of therapeutic good, for example: a medical device
- not be regulated as a therapeutic good, for example it may be a food or a cosmetic or declared to be excluded from therapeutic goods legislation.
For more information see Understanding complementary medicine interface issues and the online guidance tool: Is my product a therapeutic good? to help you determine whether a particular product is a therapeutic good, and if so, the type of therapeutic good that it is likely to be.
Important:
It is the sponsor’s responsibility to ensure that their product is a therapeutic product and required to be included in the ARTG. If you believe your product is included in the ARTG when it is not required to be, you should request cancellation of the ARTG entry.
The Secretary of the Department of Health, Disability and Ageing (the Secretary) (through the TGA) can, under section 9F of the Act, remove a product from the ARTG if satisfied that the goods are not 'therapeutic goods' as defined in the Act.
Some examples of goods at the medicine interface are provided in Table 2.
| Medicine interface – types of goods | ||
|---|---|---|
| Goods regulated as therapeutic goods | ||
| Medicines | Products that meet the definition of a therapeutic good as per the Act (and are not otherwise excluded or declared to not be a therapeutic good) are regulated as therapeutic goods. | E.g. vitamin and mineral supplements |
| Medical devices | Medical devices differ from medicines in that the principle intended action is typically achieved by physical means. Medical devices are defined by section 41BD of the Act and the Therapeutic Goods (Medical Devices - Specified Articles) Instrument 2020. Refer to Is my product a medical device? to help determine if your good is a medical device. | E.g. bandages, head lice comb |
| Boundary products | Products with components that have more than one therapeutic effect can potentially fit within more than one category of therapeutic goods are commonly referred to as ‘combination products’. These goods are regulated as either medical devices or as medicinal products, depending on the primary intended purpose of the product. For more information refer to Understanding rules for boundary and combination products. | E.g. a dressing impregnated with a medicine |
| Goods declared to be therapeutic goods | The Secretary can declare that particular goods or classes of goods are therapeutic goods - refer to Therapeutic Goods (Declared Goods) Order 2019. | E.g. products containing fibre in capsule, tablet or pill form, certain sports supplements |
| Exempt goods | Schedule 5 of the Regulations ‘exempts’ certain therapeutic goods from the requirement to be included in the ARTG (Parts 3-2 of the Act). While ‘exempt’ therapeutic goods do not need to be included in the ARTG, these products are still considered to be therapeutic goods and therefore must comply with all other applicable legislative requirements for therapeutic goods e.g. advertising and labelling requirements. See Medicines exempt from certain TGA regulation. | E.g. homoeopathic preparations more dilute than one-thousand-fold dilution of a mother tincture |
| Goods not regulated as therapeutic goods | ||
| Food | In general, if there is an applicable Australia/New Zealand Food Standard for a good or it has a history of use as a food in the way it is presented, it does not fall under the definition of a therapeutic good in the Act. Refer to Know how food and medicine are regulated and the Food-Medicine Interface Guidance Tool for information to help determine if a good is a food or a therapeutic good. | E.g. Electrolyte drinks |
| Cosmetics | Most cosmetic products are generally not considered therapeutic goods, as they tend not to be for a therapeutic use. However, a cosmetic may be a therapeutic good (medicine), depending on its ingredients, route of administration and if therapeutic claims are made on the product label or in advertising. Some cosmetics have been declared not to be therapeutic goods under the Therapeutic Goods (Excluded Goods) Determination, 2018 (see below). Refer to the AICIS website: Cosmetics and therapeutic goods to help determine if a good is a cosmetic. | E.g. Hair dye, nail varnish, perfumes |
| Goods declared not to be therapeutic goods (excluded goods) | The Secretary can declare that particular goods or classes of goods are not therapeutic goods. For the list of excluded goods, refer to: Excluded goods orders. | E.g. Tinted foundations, lip balms with sunscreen |
Types of ingredients in listed medicines and registered complementary medicines
Table 3 lists the different ingredient types/roles for ingredients included in listed and registered complementary medicines.
| Ingredient type | Explanation |
|---|---|
| Active ingredients | Regulation 2 of the Regulations states that an active ingredient, for a medicine, means a therapeutically active component in the medicine’s final formulation that is responsible for its physiological or pharmacological action. |
| Excipient ingredients | An excipient ingredient is not therapeutically active and does not contribute to the physiological or pharmacological action within the medicine’s final formulation. Types of excipient ingredients include: a fragrance, flavour, preservative, printing ink, antioxidant (protecting the formulation from oxidation), coating, binding agent, filler, an anticaking agent. Sponsors should ensure that the role of an excipient ingredient is appropriate and in an appropriate quantity for this purpose within the product formulation. Note that indications cannot be made for excipient ingredients. |
| Active Herbal Extracts | An ‘Active Herbal Extract’ (AHE) is an herbal extract or concentrate for which a supplier intends specific information on the extraction method, steps and/or solvent details, to remain confidential from sponsors who include the extract as an active ingredient in a medicine. To be a permitted ingredient in listed medicines, the active ingredient/s must comply with the definition of a herbal substance (as defined in Regulation 2 of the Regulations). Prior to November 2017, suppliers notified the TGA of details of AHEs and these were entered in the TGA Proprietary Ingredient Table. The TGA no longer issues proprietary ingredient numbers for AHEs. Ingredient suppliers can still sell herbal extracts to sponsors for inclusion as ingredients in medicines, but instead of selecting the AHE ingredient from the Proprietary Ingredient Table, sponsors include the individual ingredients (in the AHE) in their medicine application at the same time as they enter the rest of the formulation details. Some existing AHEs (included in existing medicines prior to November 2017) still remain visible in the Proprietary Ingredient Table and are able to be selected in applications for listed medicines. Sponsors with existing AHEs in their medicines’ formulation can choose to voluntarily update their ARTG entry to specify the ingredients in the formulation (instead of the AHE name and identification number) by making a correction to their ARTG entry. Standard fees and processes apply. For more information see Streamlining proprietary ingredient categories. |
| Proprietary ingredients | Proprietary ingredients consisting of excipient formulations include fragrances, flavours, colouring ingredients, trans-dermal patch adhesives and printing inks. Proprietary ingredients are entered in the TGA Business System by the TGA following submission of a Notification of a new proprietary ingredient form by the ingredient supplier. This allows for the capture of complex formulation details and other relevant information and the proprietary ingredient to be allocated a unique name and number. Sponsors may select proprietary ingredients using the assigned ingredient ID number for use in their application for a listed or registered complementary medicine. If a proprietary ingredient is to be used in a listed medicine, all ingredients within the formulation must be included in the Therapeutic Goods (Permissible Ingredients) Determination and meet the requirements of that Determination. Proprietary Ingredient suppliers are also expected to provide sufficient information to sponsors about ingredients with quantity restrictions or other relevant regulatory requirements so that sponsors are able to ensure compliance of their finished product. For further information on proprietary ingredients refer to:
|
Table 4 provides additional information on specific types of substances/ingredients in listed medicines and registered complementary medicines.
| Ingredient type | Explanation |
|---|---|
| Australian native and endangered species in complementary medicines | For queries regarding the importation of restricted/endangered species and the general importation of plant material, please refer to the following authorities: |
| Genetically modified substances | It is the responsibility of sponsors including genetically modified substances in their medicine to ensure they comply with the provisions of all relevant legislation - refer to the Office of the Gene Technology Regulator. |
| Ingredients of animal origin | Ingredients derived from animal materials may present a safety risk to consumers, as they may contain certain viruses and/or agents capable of carrying Transmissible Spongiform Encephalopathies (TSEs). Information on the TGA’s approach to minimising the risks associated with ingredients of human or animal origin is available in Demonstrating the safety of adventitious agents. There are some low-risk materials that are eligible for self-assessment. The criteria for self-assessment are outlined in Transmissible Spongiform Encephalopathies (TSE): TGA approach to minimising the risk of exposure. If the ingredient is not eligible for self-assessment, pre-clearance of animal derived ingredients should be sought from TGA before making a medicine application - refer to Pre-clearance application for animal-derived ingredients. |
| Amino acid chelates | The TGA defines a metal amino acid chelate as a complex consisting of a metal ion with one or more proteinogenic amino acid ligands bound to it in such a way that the metal ion is part of a ring within the molecule. Currently a number of metal amino acid chelates are included in the Therapeutic Goods (Permissible Ingredients) Determination and eligible for inclusion in medicines listed on the ARTG. |
Approved terminology for listed medicines and registered complementary medicines
The TGA has developed and maintains approved terminology (e.g. names) to ensure accuracy and consistency of the information about medicines in the ARTG. These approved terms are to be used in:
- applications to register or list a medicine in the ARTG
- labels and packaging for medicines
- product information documents provided with medicines
- consumer medicine information documents provided with medicines.
Approved terminology for product characteristics
Australian approved terms have been created to describe the way a medicine is presented to ensure consistency in product applications and on medicine labels, for example routes of administration, dosage forms. For more information see Other terminology. These terms are located in the Code Tables on the TGA Business Services (TBS) website.
Approved terminology for medicine ingredients
Ingredients are divided into three main categories:
- Chemical (including antibiotics)
- Biological (other than antibiotics) that are not derived from plants, algae, yeast or fungi
- Herbal, including herbal components of plant, algal, yeast or fungal origin.
Guidance on Australian approved terminology is provided in the publication TGA approved terminology for therapeutic goods. Ingredient names included in the Therapeutic Goods (Permissible Ingredients) Determination are consistent with TGA approved terminology for therapeutic goods.
Approved for new Australian approved name
When submitting an application for evaluation of a new medicine substance (including a new substance in a proposed registered complementary medicine) that does not have an approved name, the applicant should submit a proposal for a new name with that application using the appropriate form - see Proposed Australian Approved Name (AAN) application form. Name applications often occur before or in conjunction with an application for a new substance in listed medicines.
Important:
Note that assignment of a name does not imply any recommendation for the use of the substance or that the ingredient is approved for use in therapeutic goods.
TGA Business Services (TBS) Ingredients Table
The Ingredients Table located on the TBS website is a searchable database of approved terminology for chemical, biological and herbal ingredients, including:
- active ingredients
- excipients
- components and equivalents of ingredients.
This table will only provide the approved name or synonym for the ingredient. Instructions on searching this table are provided below.
Searching for ingredients via the TGA Business Services website
- Go to TGA Business Services databases and select ‘Ingredients’.
- Enter the ingredient name or synonym you are looking for in the ‘search field’ and ensure that in ‘all fields’ is selected. Click ‘Go’.
- When looking at the search results, the right-hand column will indicate if the ingredient is ‘listable’ (that is, if the ingredient is able to be included in listed medicines). The ingredient summary must be checked to determine in what context the ingredient is listable (Step 4).
- To the left of each ingredient is a down arrow icon. Click on this icon to reveal the ‘Ingredient summary’. The ‘Ingredient summary' provides the approved role of the ingredient (active or excipient).
Information on ‘Product warnings, amongst other things, can be accessed via “Code Tables’ under ‘Public TGA Information’ on the left-hand menu.
Important:
The Ingredients Table is a database and is not legislation. Sponsors should not solely refer to the Ingredients Table when formulating a medicine. The eligibility for listing and the regulatory requirements for each ingredient must be considered by consulting the Therapeutic Goods (Permissible Ingredients) Determination.
Supplying medicines in Australia
The pathway for supply of medicines in Australia varies depending on whether your product:
- is exempt from certain TGA regulation
- requires entry in the ARTG.
Medicines exempt from certain TGA regulation
Some medicines do not need to be entered in the ARTG or may be exempt from some regulatory requirements as a result of a specific exemption or determination under the Act (refer to section 18 of the Act and Schedules 5 and 5A of the Regulations), but they may be subject to:
- specific eligibility criteria (Refer to Schedule 5 of the Regulations)
- some regulatory obligations (refer to Schedule 5A of the Regulations)
Examples of exempt products include:
- Medicines (other than those used for gene therapy, that are medicinal cannabis products or that contain glucagon-like peptide-1 (GLP-1) receptor analogues) that are dispensed or extemporaneously compounded for a particular person, for therapeutic application, to that person - refer to Exemptions from inclusion in the ARTG for extemporaneously compounded medicines and dispensed complementary medicines.
- Certain homoeopathic preparations - refer to Homoeopathic preparations exempt/required to be included in the ARTG.
- Certain lotions, shampoos or hairdressings for the treatment/prevention of dandruff.
- Starting materials that are ingredients or components used in the manufacture of therapeutic goods, except when pre-packaged for supply for other therapeutic purposes or formulated as a dosage form.
These goods are exempt from Part 3-2 of the Act, relating to inclusion in the ARTG, however it is important to note that all other applicable requirements under the Act and the Regulations must be complied with, including:
- all applicable standards
- all advertising requirements
Exemptions from inclusion in the ARTG for extemporaneously compounded medicines and medicines dispensed by pharmacists
For information:
The TGA regulates therapeutic goods, we do not regulate health practitioners or their practices. The Australian Health Practitioner Regulation Agency (Ahpra) is responsible for the registration of practitioners in Australia.
The exemptions for extemporaneously prepared medicines from Parts 3-2 and 3-2A of the Act (inclusion in the ARTG) are included in Schedule 5 (item 6) of the Regulations.
The exemptions for starting materials from Parts 3-2 and 3-2A of the Act (inclusion in the ARTG) are specified in Schedule 5 (item 9) to the Regulations.
The exemptions relating to extemporaneous compounding and dispensing apply where:
- a health practitioner prepares a medicine for an individual patient either following consultation with that particular patient; or
- to fill a prescription for that particular patient.
This allows health practitioners such as pharmacists, herbalists, naturopaths, nutritionists and homoeopaths, to prepare medicines for individual patients that do not need to be assessed or evaluated by the TGA for quality, safety or efficacy. The exemption recognises the one-off nature of such medicines and the professional training of the health practitioner to prepare a medicine for the specific needs of an individual patient.
Most herbal ingredients may be used for preparing medicines that are dispensed or extemporaneously compounded. However, access to some medicinal ingredients is restricted by State and Territory drug and poisons legislation. Depending on the level of access control, some ingredients are not available for dispensing or extemporaneous compounding by health practitioners, such as: ingredients included in Schedule 4 of the Poisons Standard which are available only on prescription from an authorised prescriber registered under a law of a State or Territory.
There are also some practitioner exemptions from manufacturing requirements - refer to Products exempt from certain manufacturing requirements.
Important:
Pre-packed (manufactured) medicines made by practitioners
The exemption from inclusion in the ARTG for extemporaneously compounded medicines does not cover situations where a health practitioner makes up medicines in advance, in anticipation of patients who may come onto the premises and ask for that medicine.
Ingredients that are either pre-packaged for other therapeutic purposes or formulated as a dosage form are subject to assessment for quality, safety and efficacy as appropriate, and are to be included in the ARTG. Unless exempt, medicines included in the ARTG need to be prepared by a person in accordance with Good Manufacturing Practice (GMP).
Exemption from certain labelling requirements for ‘practitioner dispensing only’ products
Some sponsors may choose to supply their medicines in a dispensing pack solely to complementary healthcare practitioners with the intent that these medicines are supplied by a practitioner to an individual after a consultation with that individual.
If the medicine label includes the words ‘For Practitioner Dispensing Only’, there is a provision under 8(1)(n) in the Therapeutic Goods Order No. 92 - Standard for labels of non-prescription medicines (TGO 92) that the medicine label does not require a statement of the purpose or purposes for which it is intended that the medicine be used (an indication) if:
8(1)(n)(i): the medicine is:
- supplied solely to a complementary healthcare practitioner for supply to a person after affixing by the practitioner of an instruction label on the medicine following a consultation with that person; and
- the label of the medicine includes the words ‘For Practitioner Dispensing Only’[1]
Complementary healthcare practitioner means a person described in paragraph 42AA(1)(c) of the Act:
herbalists, homoeopathic practitioners, naturopaths, nutritionists or practitioners of traditional Chinese medicine registered under a law of a State or Territory
The intent of the provision in 8(1)(n)(i) of TGO 92 is that the practitioner affixes instructions for use on the medicine label prior to giving the medicine to the person, following a consultation with that person.
‘For Practitioner Dispensing Only’ medicines must meet all applicable regulatory requirements as for any other medicine included in the ARTG. This includes that labelling must meet the requirements of TGO 92 and the Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021 (the Advertising Code) and they must not refer to restricted representations (unless the appropriate exemption or approval to do otherwise has been granted).
While listed medicines that are ‘For practitioner dispensing only’ can only include ingredients permitted for use in listed medicines, sponsors may choose to formulate these medicines differently to similar types of listed medicines that are able to be self-selected by consumers at retail stores.
Products exempt from certain manufacturing requirements
Some medicines or persons are exempt from the manufacturing requirements set out in Part 3-3 of the Act. The criteria for manufacturing exemptions are provided in Section 34 of the Act, together with Schedule 7 (exempt medicines) and Schedule 8 (exempt persons) of the Regulations. Table 5 lists different products exempt from certain manufacturing requirements. For more information on GMP requirements refer to Assessing suppliers and material used in listed and complementary medicines.
| Exemption type | Description |
|---|---|
| Manufacturing exemptions for starting materials | Schedule 7 of the Regulations provides for certain ingredients used in the manufacture of therapeutic goods to be exempt from the operation of Parts 3-3 of the Act. For example: the Australian manufacturer of a ‘bulk’ essential oil (the farmer extracting oil from lavender plants) does not need to be licensed for Good Manufacturing Practice. However, the Australian manufacturers who undertake steps in the manufacturing of the finished dosage form (such as filling, blending, testing, labelling and release for supply) are required to hold the appropriate GMP licence. Note that ingredients that are either pre-packaged for other therapeutic purposes or formulated as a dosage form are subject to the therapeutic goods legislation and are required to be compliant with GMP. |
| Manufacturing exemptions for practitioners | Schedule 8(4) of the Regulations provides an exemption for specified practitioners from the operation of Part 3-3 of the Act (Manufacturing of therapeutic goods) and therefore the requirement to manufacture certain medicines under GMP: "where the preparation is for use in the course of his or her business and:
|
| Manufacturing exemptions for homoeopathic preparations | In Australia, homoeopathic medicines that:
are exempt from the Australian requirement that the manufacturer must hold a GMP license - refer to Item 7 of Schedule 7 to the Regulations. |
| Manufacturing requirements/exemptions for proprietary ingredients | Australian manufacturers who are involved in the manufacture of active ingredients, mixtures containing active ingredients and any other step taken to bring therapeutic goods to their final state (for example: intermediate manufacturing steps, testing, packaging/labelling and release for supply) are required to have a licence (TGA GMP licence) under Part 3-3 of the Act, unless specifically exempted. Where a proprietary ingredient formulation is intended to have an active role in the product formulation (e.g. a proprietary ingredient with an active and excipient ingredient/s), the manufacture of the proprietary ingredient formulation may be considered a step in the manufacture of the finished product and a TGA GMP licence or approval of the manufacturer may be required.[2] However, a proprietary ingredient formulation that has an excipient formulation with an excipient role in the medicine (for example: colours, printing inks, flavours, fragrances, and preservatives) does not require a TGA GMP licence to be manufactured. For more information on GMP requirements refer to Assessing suppliers and material used in listed and complementary medicines. |
Homoeopathic preparations exempt/required to be included in the ARTG
One consideration of whether a homoeopathic medicine is required to be included in the ARTG is the dilution (the ‘potency’) of the preparation. Homoeopathic potencies are explained below.
The expressions of homoeopathic potencies
'nX' (or 'D') potency: where each dilution of the mother tincture is a decimal or 10-fold dilution and 'n' is the number of dilutions, such that the total dilution is 10n. For example: a 1X potency represents a 1:10 dilution; 2X a 1:100 dilution; 3X a 1:1,000 dilution; 4X a 1:10,000 dilution.
- 'nC' potency: where each dilution of the mother tincture is a centesimal or 100-fold dilution and 'n' is the number of dilutions, such that the total dilution is 100n. For example: a 1C potency represents a 1:100 dilution; 2C a 1:10,000 dilution.
- 'M' potency: refers to a homoeopathic preparation that has undergone 1,000 potentisation steps in the centesimal scale. For example: 1M potency represents a 1,000C dilution; 2M a 2,000C dilution.
- 'nLM' (or 'Q'): where each dilution from a mother tincture first potentised to a 3C starting material is a quinquagintamillesimal or 50,000-fold dilution and 'n' is the number of dilutions, such that the total dilution is 50,000n. For example: a LM/01 (or Q/01) potency represents a 1:50,000 dilution from a 3C starting potency; LM/02 (or Q/02) a 1:50,000 dilution from a LM/01 (or Q/01) potency, and so on.
Specific labelling requirements for homoeopathic preparations
All commercially supplied homoeopathic medicines in Australia, regardless of whether that are/are not included in the ARTG, must:
- comply with advertising requirements set out in Schedule 2 of the Regulations
- be labelled in compliance with the general requirements for labels and the specific requirements for homoeopathic medicines in the Therapeutic Goods Order No. 92 – Standard for labels of non-prescription medicines (TGO 92) and any other applicable default standards.
Homoeopathic medicines not required to be included in the ARTG
Where a medicine meets the definition of ‘homoeopathic preparation’ and meets the conditions set out under Item 8 of Schedule 5 to the Regulations it is exempt from the requirement to be included in the ARTG.
Note that this does not apply where the homoeopathic preparation is part of a medicine containing other ingredients requiring inclusion in the ARTG.
Schedule 5 Item 8
the following goods, unless the goods are for the treatment, cure, prevention, diagnosis or monitoring of, or testing susceptibility of persons to, a disease, condition, ailment or defect:
- homoeopathic preparations more dilute than a one thousand fold dilution of a mother tincture and which are not required to be sterile;
and which do not include an ingredient of:
- human origin; or
- animal origin, if the ingredient consists of, or is derived from, any of the following parts of cattle, sheep, goats or mule deer:
- adrenal;
- brain;
- cerebrospinal fluid;
- dura mater;
- eye;
- ileum;
- lymph nodes;
- pineal gland;
- pituitary;
- placenta;
- proximal colon;
- spinal cord;
- spleen;
- tonsil;
Most homoeopathic preparations that are more dilute than a 1,000-fold dilution of a mother tincture (4X and above), are not required to be in the ARTG as they are considered to be sufficiently low risk, providing that the preparation:
- is not required to be sterile
- does not include an ingredient of human or specified animal origin
- is not for the treatment, cure, prevention, diagnosis or monitoring of, or testing susceptibility of persons to, a disease, condition, ailment or defect.
Indications (health claims) for homoeopathic medicines cannot refer to, or imply the prevention, cure or alleviation of any disease, ailment, defect or injury. However, low level therapeutic indications such as relieving symptoms of a condition are generally permitted.
Homoeopathic medicines required to be included in the ARTG
Items 4A and 5 of Schedule 4 to the Regulations provide that the following homoeopathic preparations are required to be listed in the ARTG:
- 1000 fold or lesser dilution of a mother tincture (3X or below)
- preparations more dilute than 1000 fold dilutions that make therapeutic indications.
Schedule 4 Item 4A
homoeopathic preparations where:
- the preparation consists of, or contains a dilution of, mother tincture that is a 1,000 fold dilution, or a lesser dilution, of that mother tincture; and
- the preparation only contains ingredients that are specified in a determination under paragraph 26BB(1)(a) of the Act; and
- if a determination under paragraph 26BB(1)(b) of the Act specifies requirements in relation to ingredients being contained in the preparation—none of the requirements have been contravened; and
- the preparation is not required to be sterile; and
- the preparation does not contain a substance (other than one that is more than a 1,000-fold dilution of mother tincture) included in a Schedule to the Poisons Standard; and
- the preparation only has indications that are covered by a determination under paragraph 26BF(1)(a) of the Act; and
- if a determination under paragraph 26BF(1)(b) of the Act specifies requirements in relation to the indications—none of the requirements have been contravened
Schedule 4 Item 5
homoeopathic preparations where:
each dilution is more dilute than a 1,000 fold dilution of mother tincture; and
the preparation only contains ingredients that are specified in a determination under paragraph 26BB(1)(a) of the Act; and
if a determination under paragraph 26BB(1)(b) of the Act specifies requirements in relation to ingredients being contained in the preparation—none of the requirements have been contravened; and
the preparation is not required to be sterile; and
the preparation only has indications that are covered by a determination under paragraph 26BF(1)((a) of the Act; and
if a determination under paragraph 26BF(1)(b) of the Act specifies requirements in relation to the indications—none of the requirements have been contravened
Homoeopathic preparations that are listed in the ARTG must meet the following requirements:
- only contain ingredients specified in the Therapeutic Goods (Permissible Ingredients) Determination
- only contain indications covered by the Therapeutic Goods (Permissible Indications) Determination
- not be required to be sterile
- not contain a substance included in a Schedule to the Poisons Standard [other than one that is more than a 1,000 fold dilution of mother tincture (4X and above)]
Entering medicines in the ARTG
If your product is a therapeutic good and is not exempt or excluded, then you must apply to include your medicine in the ARTG. To include a medicine in the ARTG an applicant will need to:
Identify the appropriate approval pathway (L, L(A) or R) for the medicine. Understand the non-prescription medicine pathways assists potential sponsors of medicines to determine the most suitable pathway to enter their product in the ARTG.
Apply to enter the medicine in the ARTG using the applicable form for the appropriate application type.
Determining the ARTG entry pathway
The ARTG entry pathways for medicines are based on the ingredients they contain and their therapeutic indications (intended use). Medicines can be listed, assessed listed or registered in the ARTG (note that in relation to registered medicines, this guidance refers to registered complementary medicines).
Table 6 summarises the key aspects and regulatory requirements for each ARTG entry pathway.
Table 7 outlines the risk categories of medicines based on the claimed therapeutic indications.
| Listed medicines | Assessed listed medicines | Registered complementary medicines | |
|---|---|---|---|
| ARTG | AUST L | AUST L(A) | AUST R |
| GMP | All medicines be manufactured in accordance with the principles of GMP. | ||
| Sterility | Must not be required to be sterile. | May or may not be required to be sterile depending on medicine type. | |
| Ingredients | May only include ingredients in the Therapeutic Goods (Permissible Ingredients) Determination. |
| |
| Indications | Can only have indications in the Therapeutic Goods (Permissible Indications) Determination. |
| May have:
|
| Evidence | Evidence held by the sponsor. Not assessed pre-market by the TGA. | Quality and safety evidence held by the sponsor. Efficacy evidence must be assessed pre-market by the TGA. | Safety, quality and efficacy evidence must be assessed pre-market by the TGA. |
| Application and TGA pre-market evaluation | Sponsor self-certification safety, quality and efficacy. No pre-market evaluation. | Sponsor self-certification of quality and safety. TGA pre-market assessment of efficacy. | Full pre-market assessment of quality, safety and efficacy by the TGA. |
| ‘TGA assessed’ claim | Do not have option to use ‘TGA assessed’ claim. | Have the option to use a ‘TGA assessed’ claim. | Have the option to use a ‘TGA assessed’ claim. |
| Post market compliance review | All listed medicines may be selected for a post-market compliance review at any time. The TGA will check the medicine’s compliance against any regulatory requirements self-certified by the sponsor. | Continuing safety, quality and efficacy of therapeutic goods in the market monitored through the Pharmacovigilance Inspection Program. | |
| Low risk – listed medicines | Intermediate risk – Assessed listed medicines | Higher risk – Registered complementary medicines |
|---|---|---|
Permitted indications may refer to:
A low level indication must not:
| Intermediate level indication may refer to:
Intermediate level indications may include those indications specified in a non-permitted indications list (if such a list is made). An intermediate level indication must not:
| High level indications may refer to the prevention, cure or alleviation of a restricted representation. A high level indication must not:
|
For the application process for the ARTG entry pathways refer to:
- ‘Application process to list a medicine’ in the ARTG in Understanding the legislative framework for listed medicines
- ‘Application process for a registered complementary medicine’ in Submitting an application for a registered complementary medicine.
Supplying medicines packaged with other goods
Medicines may be individually packaged with other therapeutic goods. These may be defined as a kit, a composite pack, a system or procedure pack.
Kits and composite packs are defined in the legislation under section 7B of the Act. System or procedure packs are defined in section 41BF - these are regulated as medical devices. Packs are regulated differently depending on the combination of therapeutic goods supplied.
Composite pack
- Contains two or more therapeutic goods but does not contain a medical device.
- The goods must be for administration as a single treatment or as a single course of treatment, and the components are either combined before treatment or administered in a particular sequence as part of that treatment.
- Individual components within the pack are not separately listed or registered in the ARTG. That is, a composite pack has one single ARTG entry.
An example of a composite pack is a carton containing Day & Night Cold & Flu tablets (i.e. different tablets to be taken either during the day or during the night).
Kits
- Contains one or more (listed, registered or exempt) goods that are supplied in a single package.
- The pack must not meet the definition of a composite pack. The individual goods can be used independently and do not need to be combined or administered in a sequence for the product's therapeutic purpose.
- Individual therapeutic components within the pack must be separately listed, registered or excluded from the requirement to be in the ARTG.
- The kit (comprised of the individually regulated products) is included in the ARTG as a listed medicine (even if one of the goods in the kit is a registered medicine or an exempt good).
An example of a kit is a sunscreen lotion (AUST L listed medicine), and a cosmetic moisturiser (exempt good) and a sun hat (non-therapeutic good) presented together in a single package for sun care.
System or procedure pack
A system or procedure pack contains medicine/s and at least one medical device that are required to be used together as a system or in a medical or surgical procedure. System or procedure packs are regulated as medical devices.
For information:
System or procedure packs are medical devices.
Information on the regulation of system or procedure packs can be found in pre-market part of the Australian Regulatory Guidelines for Medical Devices.
Administration information
Fees and charges
The TGA has a variety of fees and charges for complementary medicines:
- an initial application fee
- evaluation fees
- an annual charge to maintain the inclusion of their product in the ARTG.
The fees payable varies depending on the type of application. For more information refer to the Summary of fees and charges to applications submitted to the TGA.
Appeals processes
If the sponsor does not agree with a decision made under the Act by the TGA, the Act provides a comprehensive system for review of administrative decisions. The appeal mechanisms are described in more detail in the Requesting the Minister for Health to reconsider our initial review. Briefly, the formal appeal process usually involves:
- An appeal under Section 60 of the Act.
This can be followed by:
Post-market monitoring of medicines in Australia
Important:
Section 29A of the Act requires sponsors of medicines registered or listed in the ARTG to report adverse reactions about which they become aware. Guidance for sponsors is provided in Pharmacovigilance responsibilities of medicine sponsors.
We adopt a risk management approach to regulating therapeutic goods including post-market monitoring activities. Information on our approach to managing compliance risk is available at: Our regulatory framework.
The TGA has a multi-faceted program for monitoring the safety, quality and efficacy of therapeutic products that are on the market. The types of post market regulatory activities we undertake include:
- Pharmacovigilance Inspection Program
- Report an adverse event or safety problem
- Understanding listed medicines compliance reviews
- Compliance management
- TGA Laboratories: What we test, how we test and what we do with our results
- Good manufacturing practice: An overview.
Once a problem has been identified, possible regulatory actions are undertaken by the TGA which can include:
- Informing health practitioners and consumers about the risks of using the product.
- Re-assessing the benefit-risk profile.
- Requiring product labelling changes.
- Requiring design or manufacturing change.
- Removal of the product from the ARTG.
- Recalling products - refer to: Procedure for recalls, product alerts and product corrections (PRAC).
Footnotes
[1] In many places in the TGO 92 allows label statements to be followed with “or words to that effect”. In the case of 8(1)(n)(i)(B), TGO 92 does not give that option. That conveys that only those precise words meet the requirements of TGO 92.
[2] The TGA no longer issues proprietary ingredient numbers for ingredient mixtures that contain an active ingredient. For more information see Streamlining proprietary ingredient categories.
[3] Excludes approved restricted representations for sunscreens, folic acid, vitamin D and calcium.
[4] A restricted representation is any reference expressly or by implication, to a serious form of a disease, condition, ailment or defect identified in section 28 of the Therapeutics Goods Advertising Code. It is generally accepted to be beyond the ability of the average consumer to diagnose, evaluate, and/or safely treat without consulting a suitably qualified health care professional, however, excludes conditions where the serious form has been medically diagnosed and medically accepted as being suitable for self-treatment and management. Prior TGA approval is required before an advertisement may refer to a restricted representation.
Page history
- Title change from ‘Overview of the regulation of listed medicines and registered complementary medicines’ to ‘Understanding listed and registered complementary medicine regulation’, and formatting changes to align with ‘Guidance’ content type.
- Update to the wording for the labelling of products intended ‘For practitioner dispensing only’, including to include the term ‘complementary’ healthcare practitioners.
- Updates to URLs linking to TGA and other websites. Updating the names of organisations, web pages and guidance materials.
- Update of the definitions of traditional medicines, herbal substance, homoeopathic and anthroposophic medicines to reflect legislative definitions. Update definition of essential oils to reflect definition in the TGA Code Tables.
- Update of information in Table 2: Types of goods at the medicine interface.
- Update of Table 7: Risk-based hierarchy of indications to be consistent with Assessed listed medicines evidence guidelines.
- Update to the sections on composite packs and kits.
- Update to the homoeopathic preparations sections to include information about homoeopathic potencies and inclusion of Items 4A and 5 from the Regulations.
Information on Active Herbal Extracts and proprietary ingredients updated.
This document, ‘Overview of the regulation of listed medicines and registered complementary medicines’, has been extracted from ARGCM v.8 April 2018 pages11 to 44 (previously named ‘ARGCM Part A’).
The document has been simplified to be an overview of the regulatory framework. The sequence of information, headings and formatting have been changed from the original content for consistency and easier navigation. References to outdated forms have been removed.
The introduction has been modified to provide an overview of the regulatory framework for medicines and explanatory information on the classification of listed and registered medicines.
New guidance has been included on changes to the regulatory framework for listed medicines, including:
- permitted indications
- the assessed listed pathway
The following technical content has been extracted to be new standalone guidance documents:
- Literature-based submissions for listed medicines and registered complementary medicines: guidance for sponsors (extracted from ARGCM Part A pages 41 -43)
- Listed medicine presentation and labels: guidance for sponsors (extracted from ARGCM v8 Part A pages 27 -29)
Information on changing listed medicines and registered complementary medicines have been moved in to the relevant new guidance for these goods.
Information on proprietary ingredients clarified and links provided to Supplier assessment, approval and qualification for listed and complementary medicines.
- Title change from ‘Overview of the regulation of listed medicines and registered complementary medicines’ to ‘Understanding listed and registered complementary medicine regulation’, and formatting changes to align with ‘Guidance’ content type.
- Update to the wording for the labelling of products intended ‘For practitioner dispensing only’, including to include the term ‘complementary’ healthcare practitioners.
- Updates to URLs linking to TGA and other websites. Updating the names of organisations, web pages and guidance materials.
- Update of the definitions of traditional medicines, herbal substance, homoeopathic and anthroposophic medicines to reflect legislative definitions. Update definition of essential oils to reflect definition in the TGA Code Tables.
- Update of information in Table 2: Types of goods at the medicine interface.
- Update of Table 7: Risk-based hierarchy of indications to be consistent with Assessed listed medicines evidence guidelines.
- Update to the sections on composite packs and kits.
- Update to the homoeopathic preparations sections to include information about homoeopathic potencies and inclusion of Items 4A and 5 from the Regulations.
Information on Active Herbal Extracts and proprietary ingredients updated.
This document, ‘Overview of the regulation of listed medicines and registered complementary medicines’, has been extracted from ARGCM v.8 April 2018 pages11 to 44 (previously named ‘ARGCM Part A’).
The document has been simplified to be an overview of the regulatory framework. The sequence of information, headings and formatting have been changed from the original content for consistency and easier navigation. References to outdated forms have been removed.
The introduction has been modified to provide an overview of the regulatory framework for medicines and explanatory information on the classification of listed and registered medicines.
New guidance has been included on changes to the regulatory framework for listed medicines, including:
- permitted indications
- the assessed listed pathway
The following technical content has been extracted to be new standalone guidance documents:
- Literature-based submissions for listed medicines and registered complementary medicines: guidance for sponsors (extracted from ARGCM Part A pages 41 -43)
- Listed medicine presentation and labels: guidance for sponsors (extracted from ARGCM v8 Part A pages 27 -29)
Information on changing listed medicines and registered complementary medicines have been moved in to the relevant new guidance for these goods.
Information on proprietary ingredients clarified and links provided to Supplier assessment, approval and qualification for listed and complementary medicines.

