Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This Guidance provides sponsors of assessed listed medicines and registered complementary medicines information on:
- what the TGA assessed claim is,
- how the TGA assessed claim can be used on medicine labels and in other advertising,
- how to display the registered trade mark, and
- how to apply to use the TGA assessed claim.
Please note the examples of product labels in this document are intended to provide information on the presentation of the TGA assessed claim only. They do not represent actual products, nor necessarily meet the requirements of the therapeutic goods labelling order or advertising requirements.
For information on requirements for labelling refer to Labelling and packaging and for information on advertising refer to the Advertising hub on the TGA's website.
Legislation
The TGA assessed claim
The TGA assessed claim can be used to indicate that a medicine has had the efficacy of its indications assessed by the TGA. The claim must only be used in relation to assessed listed medicines or registered complementary medicines.
The claim must be displayed as the approved TGA assessed statement, with or without the approved TGA assessed symbol.
The TGA assessed claim is not a recommendation by the TGA and does not advocate that a medicine with the claim is better for a person than other medicines without the claim.
The TGA assessed claim is not mandatory, and therefore not all medicines whose efficacy has been assessed may display it.
The TGA assessed symbol is a certification trade mark in the Register of Trade Marks under the Trade Marks Act 1995. The ® symbol or the words “registered trade mark” signifies that the trade mark is protected and prevents others from using it without our permission or using a similar mark that could cause confusion for consumers.
Sponsors may choose to include the wording “The TGA Assessed trade mark is a registered certification trade mark owned by The Crown in Right of the Commonwealth of Australia, Department of Health and Aged Care, Therapeutic Goods Administration and used by [name of company] under licence.” but are not compelled to. However, sponsors are encouraged to include the ® symbol or the word “registered trade mark” next to the TGA assessed symbol however there is no legal obligation to do so.
What medicines it applies to
Assessed listed medicines and registered complementary medicines which have undergone a pre-market assessment of efficacy by the TGA are eligible to use the TGA assessed claim on their medicine label and other advertising material.
Assessed listed medicines are different to other listed medicines.
Sponsors of listed medicines (with an 'AUST L' ARTG number) are required to hold evidence for the efficacy of their listed medicine. However, this evidence is not pre-market assessed by the TGA.
Assessed listed medicines (with an 'AUST L(A)' ARTG number) have undergone a TGA pre-market assessment of the efficacy of the medicine's indications.
Listed or registered complementary medicines that have not undergone pre-market efficacy assessment by the TGA are not eligible to use the TGA assessed claim. For example, grandfathered registered complementary medicines will not be eligible to use the TGA assessed claim unless the sponsor applies to the TGA to complete an assessment of the medicine's efficacy. Only medicines with indications supported by scientific evidence and approved for inclusion by the TGA will be eligible to use the TGA assessed claim.
Prescription and over the counter (OTC) medicines will not display the TGA assessed claim.
The TGA assessed symbol and statement are shown below.
The TGA assessed symbol
The TGA assessed symbol

An example of the TGA assessed symbol with the registered trade mark symbol in the top righthand corner for use on labels for assessed listed medicines and registered complementary medicines.
Example label with the TGA assessed symbol
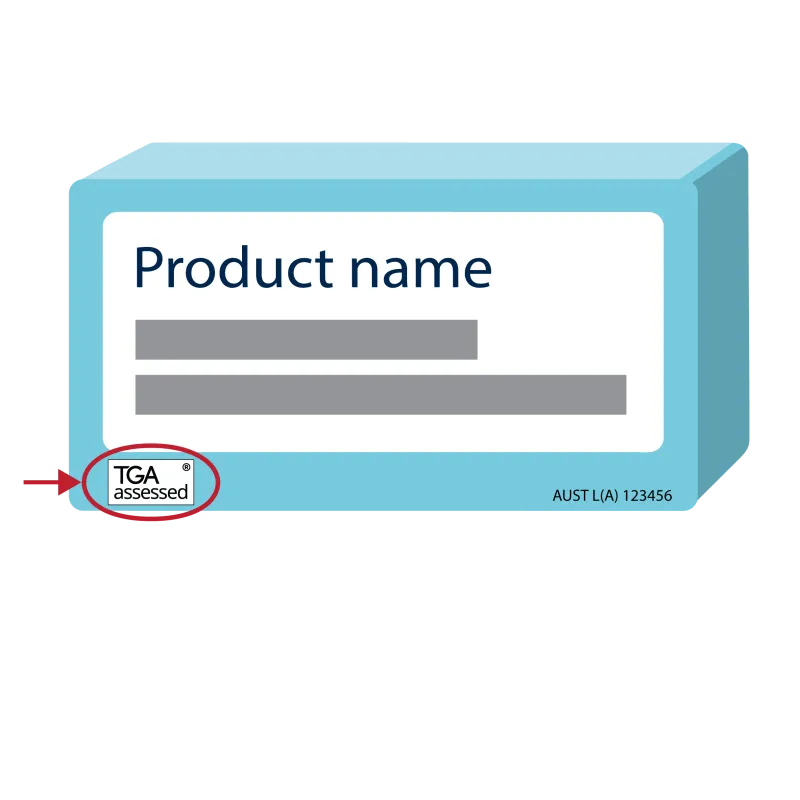
An illustrated example of medicine packaging with the TGA assessed symbol, with the registered trade mark symbol in the top righthand corner, displayed on the bottom lefthand corner of the box.
If the symbol is used, a statement authorised by the TGA must also be used (see below).
The TGA assessed statement
The statement may be used on its own without the TGA assessed symbol. The wording of the TGA assessed statement must not be changed.
Examples of compliant and non-compliant statements
Compliant: Evidence for the approved indications has been assessed by the TGA.
Non-compliant: The TGA has assessed our evidence and has approved all of our indications.
Example medicine label with TGA assessed statement
Example label with statement
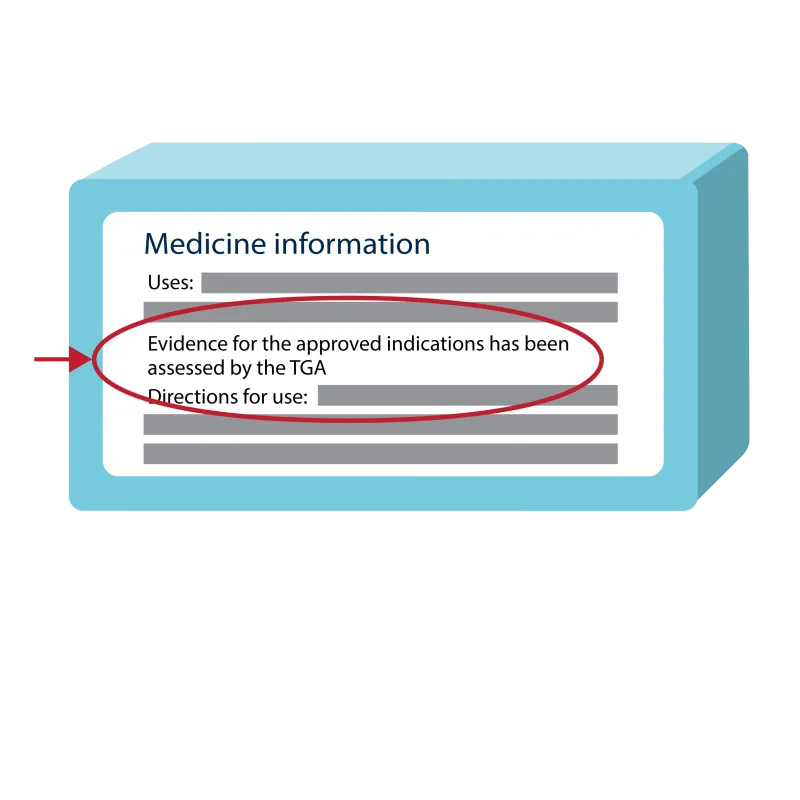
An illustrated example of medicine packaging with the TGA assessed statement on the box. The statement reads, "Evidence for the approved indications has been assessed by the TGA."
Using the TGA assessed claim on product labels
The TGA assessed claim may only be used in relation to a medicine when that use has been authorised by the TGA.
Authorisation to use the TGA assessed claim will only be issued for assessed listed medicines and registered complementary medicines that have undergone a pre-market assessment of efficacy by the TGA.
The TGA assessed claim must be easily identifiable on medicine labels. The label of the medicine that will display the TGA assessed claim is also assessed as part of this process.
The TGA assessed symbol and statement are standardised for easy identification on different product types. The requirements for using the TGA assessed claim on a medicine label are provided below.
Location of the TGA assessed claim on a product label
The TGA assessed claim (the TGA assessed statement, with or without the TGA assessed symbol) can be displayed anywhere on the label, including the primary pack.
The TGA assessed claim must be balanced against other information on the main label and primary pack to assist consumers in selecting and using a medicine.
The size of the TGA assessed claim must not be more prominent than required label information.
For more information on labelling requirements refer to TGO 91 and TGO 92.
Section 3(1) of the Therapeutic Goods Act 1989 provides the following definitions of primary pack and label:
primary pack, in relation to therapeutic goods, means the complete pack in which the goods or the goods and their container, are to be supplied to consumers.
label, in relation to therapeutic goods, means a display of printed information:
(a) on or attached to the goods; or
(b) on or attached to a container or primary pack in which the goods are supplied; or
(c) supplied with such a container or pack.
Text size of the TGA assessed claim
TGA assessed symbol
Minimum text size for the TGA assessed symbol

The minimum text size for the letters in the word 'assessed' included in the TGA assessed symbol is 1.5 millimetres, inclusive of the ascender. Therefore, the minimum overall size of the symbol/information box is equivalent to 4.7 mm high and 9 mm wide. The registered trade mark symbol needs to be displayed in the top righthand corner as superscript.
Diagram showing minimum size requirements for the TGA assessed symbol on medicine labels in Australia.
The symbol is a rectangular box containing the text "TGA assessed" in two lines.
The registered trade mark symbol is displayed in the top righthand corner.
Overall dimensions are 9 mm wide by 4.7 mm high.
The text "assessed" has a minimum height of 1.5 mm for lowercase letters, measured to the top of ascenders.
Maximum text size for the TGA assessed symbol
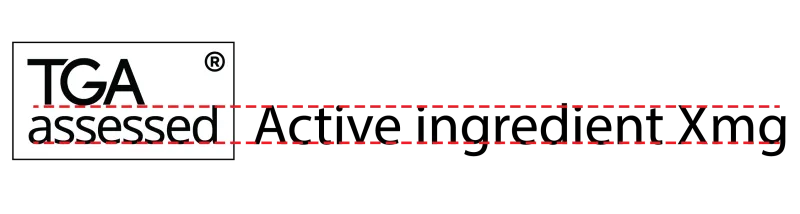
The maximum text size for the word ‘assessed’, inclusive of the ascender, is no more than the text size of the letters for the active ingredients, warnings or other essential information included on the medicine label. The registered trade mark symbol needs to be displayed in the top righthand corner as superscript.
Diagram showing text size comparison for TGA assessed symbol on medicine labels.
The registered trade mark symbol is displayed in the top righthand corner.
The text 'TGA assessed' is shown, with a dotted red line indicating the maximum height of the word 'assessed'.
The line extends to text reading 'Active ingredient Xmg', demonstrating that the 'assessed' text should not exceed the height of critical information like active ingredients on the label.
TGA assessed statement
The minimum text size for the TGA assessed statement is 1.5 millimetres.
The maximum text size for the TGA assessed statement is no more than the text size for the names and amounts of active ingredients, warnings or other essential information included on the medicine label.
Font style and colour of TGA assessed claim
TGA assessed symbol
The TGA assessed symbol must be presented in black ink on a white background and surrounded by a black outline (as provided to the sponsor in an .eps file by the TGA). The Registered trade mark symbol needs to be displayed in the top righthand corner in superscript.
Included below are examples of compliant and non-compliant TGA assessed symbols.
Example of a compliant TGA assessed symbol

Compliant: Do present the symbol in black with a white background and a black outline.
An example of a compliant TGA assessed symbol, which presents the TGA assessed symbol (including the registered trade mark symbol in the top righthand corner) in black with a white background and a black outline.
Example 1 of a non-compliant TGA assessed symbol

Non-compliant: Do not change the colour of the symbol or the outline. Do not invert the symbol.
An illustrated example of a non-compliant TGA assessed symbol, with the TGA assessed symbol (including the registered trade mark symbol in the top righthand corner) shown in a pink font on a white background.
Example 2 of a non-compliant TGA assessed symbol

Non-compliant: The symbol must be presented on a white background.
An illustrated example of a non-compliant TGA assessed symbol, with the TGA assessed symbol (including the registered trade mark symbol in the top righthand corner) shown in a black font on a blue background.
Example 3 of a non-compliant TGA assessed symbol

Non-compliant: Do not change the shape of the symbol or any of its elements, do not stretch or skew the symbol.
An illustrated example of a non-compliant TGA assessed symbol, with the TGA assessed symbol (including the registered trade mark symbol in the top righthand corner) shown with an altered shape.
TGA assessed statement
To allow sponsors some flexibility in label design, you can choose the font style and colour for the TGA assessed statement; however, it must:
- be presented in a colour that contrasts strongly with the background it is printed on; and
- be consistent with the font style, colour and prominence of the surrounding label text, warnings or other essential information.
The sentence must be written in lower case, with the exception of the first letter of the sentence and TGA, as shown below.
Examples of compliant and non-compliant TGA assessed statements
Compliant: Evidence for the approved indications has been assessed by the TGA.
Non-compliant: EVIDENCE FOR THE APPROVED INDICATIONS HAS BEEN ASSESSED BY THE TGA.
Examples of TGA assessed (symbol and statement) on labels
Example of a TGA assessed claim (statement)
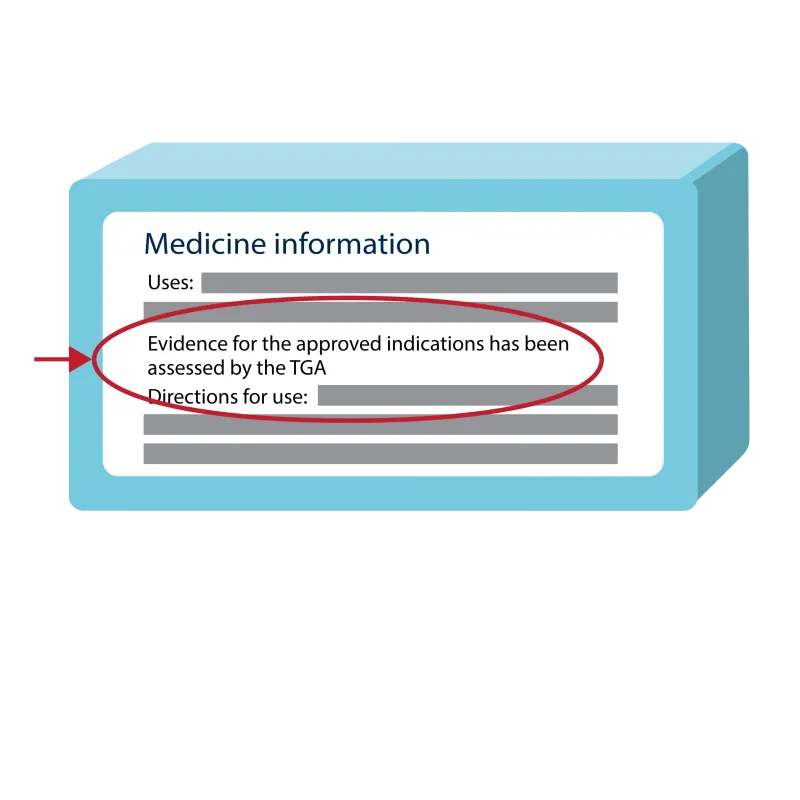
A simplified illustration of a light blue medicine box.
On the label, there's a section titled 'Medicine information'.
Below this, a line of text is highlighted with a red oval, which reads: 'Evidence for the approved indications has been assessed by the TGA'.
Example 1 of a TGA assessed claim (symbol)

A stylised illustration of a medicine bottle label with the text 'YOUR MEDICINE' prominently displayed in black on a yellow background.
Below this is smaller text reading 'INGREDIENTS'.
In the bottom right is a magnified image from the medicine bottle of the TGA assessed symbol. The TGA assessed symbol features 'TGA' in large black letters and 'assessed' in smaller black letters underneath, with the registered trade mark symbol in the top righthand corner, with a white background, indicating the product has been assessed by the Therapeutic Goods Administration.
Example 2 of a TGA assessed (symbol)

An illustrated example of a medicine package label that reads 'MediBrand Joint relief'.
The label is primarily green and white. It shows product details including '20
Capsules' and 'Active ingredient 5 mg'.
The bottom right contains an example of the TGA assessed symbol. The TGA assessed symbol features 'TGA' in large black letters and 'assessed' in smaller black letters underneath, with the registered trade mark symbol in the top righthand corner, with a white background, indicating the product has been assessed by the Therapeutic Goods Administration.
How the TGA assessed claim be used
The specific rules of how the TGA assessed claim can be used on the label and in advertising is set out in the Therapeutic Goods (TGA Assessed Claim) Authorisation 2020 (authorisation instrument). These rules apply to the sponsor of the medicine and also apply to advertisers of the medicine who are not the sponsor (e.g. secondary advertisers such as retailers or pharmacies).
How the TGA assessed claim is regulated
The Therapeutic Goods (TGA Assessed Claim) Authorisation 2020 (authorisation instrument) specifies the circumstances in which the TGA assessed claim can lawfully be used, and is made under the Therapeutic Goods Act 1989.
An authorisation to use the TGA assessed claim is required because a claim that suggests or implies that goods have been recommended, approved or endorsed by the TGA would, unless authorised, breach the Therapeutic Goods Act 1989 and the Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021.
Sponsors are required to ensure that use of the TGA assessed claim, whether on the label or otherwise, is in accordance with the authorisation instrument. Sponsors will also be required to take steps to ensure that any advertiser of its medicine also ensures that use of the TGA assessed claim is in accordance with the authorisation instrument.
Use of the TGA assessed claim other than in accordance with the authorisation instrument is likely to breach the Therapeutic Goods Act 1989 and the Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021, which may result in criminal and/or civil penalties, and/or cancellation of a medicine from inclusion in the Australian Register of Therapeutic Goods.
A sponsor seeking to use the TGA assessed claim on its label must submit the label to the TGA for approval prior to including the TGA assessed claim on its label.
Using the TGA assessed claim in other advertising materials
Advertisements may show images of the medicine label, including the TGA assessed claim (the TGA assessed statement with or without the TGA assessed symbol), but must not use the TGA assessed symbol or statement other than on the image of the label in the pack shot.
An advertisement must not include any audible commentary or further written commentary on the TGA assessed claim.
The TGA assessed claim must not be more prominent than the mandatory statements in an advertisement.
The use of the TGA assessed claim in any advertising must comply with the advertising requirements set out in the Therapeutic Goods Act 1989 and the Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021, as amended from time to time.
The advertising must not imply that the TGA recommends the medicine or advocates the use of the medicine above other medicines that do not use the TGA assessed claim.
Example of TGA assessed claim in advertising materials

An example of TGA assessed claim used in advertising. The example shows an advertisement for a digestive health product, Bean's tonic+ IBS relief supplement.
The image shows a woman with short blonde hair wearing a light blue t-shirt, holding her stomach with a pained expression.
The text reads 'GOT GAS? BE KIND TO YOUR GUTS WITH BEAN'S'.
A product bottle is displayed, labelled 'bean's tonic+ IBS relief' with green capsules beside it.
The bottle's label includes text stating 'Active Ingredient 5 mg' directly above a black and white 'TGA assessed' symbol and registered trade mark symbol in the top righthand corner.
How to access the TGA assessed claim for my assessed listed or registered complementary medicine
If a sponsor wishes to use the TGA assessed claim for a new assessed listed or new registered complementary medicine, they must provide a medicine label with the TGA assessed claim for TGA pre-market approval.
If a sponsor wishes to use the TGA assessed claim for an existing registered complementary medicine that has undergone a pre-market assessment of efficacy by the TGA, they must have their new label assessed by the TGA before the claim can be used. Applications for changes to the medicine label which only relate to the use of the TGA assessed claim can use the 'OT1' code. This will attract a RCMC1 application fee – refer to TGA's Schedule of fees and charges.
You can request the TGA assessed symbol file by emailing: nonprescriptionmedicines@health.gov.au. The file will be provided in an .eps format.
In considering a label in relation to a medicine, the TGA will consider all aspects of the medicine's presentation for compliance with the various legislative requirements.
Authorisation to use the TGA assessed claim will not be given in relation to a medicine where the presentation of that medicine (with the TGA assessed claim) is not acceptable - refer to the Australian Regulatory Guidelines for Listed Medicines and Registered Complementary Medicines for further information.
Page history
- Information about the TGA assessed symbol being a registered trade mark.
- Updated images to show the ® symbol.
- New sections added:
- What medicines does it apply to?
- How the TGA assessed claim can be used
- How the TGA assessed claim is regulated
- How to access the TGA assessed claim for my assessed listed or registered complementary medicine
- Updated contact email address
Title changed from 'Guidelines for using the TGA assessed claim on medicine labels' to 'Using the 'TGA assessed' claim on medicine labels' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
- Clarification of measurement of the text height in line with existing TGO 92 guidance.
- Revision of minimum TGA assessed symbol dimensions to allow sponsors to use a smaller symbol, if the mandatory label information text is at the minimum height required by TGO 92.
- Updated link to the Australian Regulatory Guidelines for Listed Medicines and Registered Complementary Medicines.
- Addition of information regarding certification trade mark.
Original publication
- Information about the TGA assessed symbol being a registered trade mark.
- Updated images to show the ® symbol.
- New sections added:
- What medicines does it apply to?
- How the TGA assessed claim can be used
- How the TGA assessed claim is regulated
- How to access the TGA assessed claim for my assessed listed or registered complementary medicine
- Updated contact email address
Title changed from 'Guidelines for using the TGA assessed claim on medicine labels' to 'Using the 'TGA assessed' claim on medicine labels' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
- Clarification of measurement of the text height in line with existing TGO 92 guidance.
- Revision of minimum TGA assessed symbol dimensions to allow sponsors to use a smaller symbol, if the mandatory label information text is at the minimum height required by TGO 92.
- Updated link to the Australian Regulatory Guidelines for Listed Medicines and Registered Complementary Medicines.
- Addition of information regarding certification trade mark.
Original publication

