Understanding priority applicant determination rules for medical devices including in-vitro diagnostics (IVDs)
Guidance on the priority applicant determination criteria and process for medical devices including in vitro diagnostics (IVDs).
Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is to assist applicants seeking a conformity assessment (priority applicant) determination or a medical devices (priority applicant) determination.
These guidelines generally refer to such determinations, in either case, as 'priority applicant determinations'.
About this guidance
For a medical device, including an in vitro diagnostic medical device (IVD), priority applicant determinations provide for a person to be a priority applicant in relation to either:
- an application for a conformity assessment certificate issued by us
- an application for inclusion in the Australian Register of Therapeutic Goods (ARTG).
When a priority applicant determination is made, the corresponding application for conformity assessment or ARTG inclusion will undergo priority consideration. This means that application will be allocated front-of-queue priority throughout the relevant assessment processes.
Obtaining a priority applicant determination does not, of itself, guarantee approval of the application for conformity assessment or inclusion. These applications must still be assessed against, and satisfy, the relevant legislative requirements, in order for conformity assessment certification to be issued, and/or for a kind of device to be included in the ARTG.
For more information, see Standards, guidelines & publications (medical devices & IVDs).
Priority applicant determinations cease to be in force after 6 months if no application for TGA conformity assessment or ARTG inclusion (as relevant) is made.
The making of a priority applicant determination does not guarantee approval of the application for conformity assessment or ARTG inclusion. Compliance with the Essential Principles is not assessed as part of a priority applicant determination.
The two types of priority applicant determinations
If you seek priority consideration in relation to a medical device, you only need to apply for one type of priority applicant determination.
Conformity assessment (priority applicant) determination
If you need a TGA-issued conformity assessment certificate, then the relevant priority applicant determination to apply for is a conformity assessment (priority applicant) determination.
If you obtain TGA conformity assessment, the process for inclusion in the ARTG does not require audit and typically occurs within a short period of time after the application for inclusion is lodged and the relevant application fee is paid. A separate application for a medical devices (priority applicant) determination seeking priority consideration in the inclusion process is not necessary.
Medical devices (priority applicant) determination
If you do not need a TGA-issued conformity assessment certificate (for example, you may already have overseas conformity assessment), and you seek priority consideration in the inclusion process, then the relevant priority application determination to apply for is a medical devices (priority applicant) determination.
If you do not require 'front-of-queue' priority consideration (for your conformity assessment application or inclusion application), you do not need to apply for a priority applicant determination.
Legislative framework
The relevant legislation regulating medical devices is:
- Therapeutic Goods Act 1989 (the Act) particularly Chapter 4 of the Act
- Therapeutic Goods (Medical Devices) Regulations 2002 (the Regulations).
The legislative framework for conformity assessment (priority applicant) determinations and medical device (priority applicant) determinations involves separate, but equivalent, provisions.
The Act contains framework provisions including that if a priority applicant determination is in force, it may be published on the Department's website (sections 41ECA and 41FKA, respectively).
The Regulations (in Regulations 4.3B to 4.3E, and 5.4A to 5.4D, respectively) provide for:
- application requirements (and the prescribed fee as set out in Schedule 5)
- criteria
- notification of the decision on the application for a priority applicant determination
- specific information to be set out in the priority applicant determination
- period when a priority applicant determination is in force
- revocation of priority applicant determinations.
The Regulations contain a definition for the term 'intended purpose' (which is referred to in the criteria):
Intended purpose of a medical device means the purpose for which the manufacturer of the device intends it to be used, as stated in:
- the information provided with the device; or
- the instructions for use of the device; or
- any advertising material applying to the device; or
- any technical documentation describing the mechanism of action of the device.
The Regulations also provide for applicants to request reconsideration of decisions to refuse to make, or to revoke, priority applicant determinations (Regulation 10.7).
Criteria for priority applicant determination
The Regulations set out three criteria, all of which must be satisfied in relation to the medical device (referred to in the Regulations as 'the new device'), in order to obtain a priority applicant determination.
The criteria are set out in in the table below. Note that criterion 2 and 3 contain requirements in the alternative that can be met.
| Criteria | Description |
|---|---|
| 1. Life-threatening or seriously debilitating condition | The intended purpose of the new device is the monitoring, treatment, prevention or diagnosis of a life-threatening or seriously debilitating condition. |
| 2. Unmet need/significant improvement | Either:
|
| 3. Major clinical advantage (or in the case of IVDs, 'major public health benefit') | At least one of the following must apply to the new device:
|
Addressing the criteria
You need to make a concise, persuasive argument against all 3 criteria to demonstrate eligibility for a priority applicant determination, supported by evidence including epidemiological and clinical evidence. The criterion that is most typically an issue is criterion 3.
The epidemiological and clinical evidence you provide will be assessed for quality (including for study type, size and design). As relevant, the quality of the evidence will also be relevant when comparability is an issue, for example, to existing devices, technology or alternatives in the ARTG. The quality of the evidence you provide, rather than the quantity, is important in seeking to demonstrate relevant criteria are satisfied.
Criterion 1: Life-threatening or seriously debilitating condition
This criterion requires that the intended purpose of the medical device is the monitoring, treatment, prevention or diagnosis of a life-threatening and/or seriously debilitating condition.
The term 'intended purpose' is defined in the Regulations (see the Legislative framework section above). In practical terms, the intended purpose is the proposed intended purpose for ARTG inclusion. This must be the same as the stated intended purpose at the time of the application for a priority applicant determination.
The terms life-threatening condition and seriously debilitating condition should generally be understood, and evidence provided, with reference to the type of matters noted in the glossary in relation to these terms.
In your application, for example, you should provide evidence in support of at least one of the following:
- the life-threatening nature of the condition based on figures of mortality and life expectancy in Australia
- the seriously debilitating nature of the condition based on morbidity over the course of the condition and its impact on patients' day-to-day functioning based on objective and quantifiable medical or epidemiologic information. Where the evidence is that morbidity is short-lived and/or self-limiting, as a general position this would not support there being a seriously debilitating condition.
Criterion 2: Unmet need/significant improvement
This criterion can be met in one of two ways:
- if there are no devices with that intended purpose (being for the monitoring, treatment, prevention or diagnosis of a life-threatening or seriously debilitating condition) of a kind included in the ARTG
- if there are existing devices with that intended purpose of a kind included in the ARTG - there must be substantial evidence demonstrating that the safety or performance of the new device when used for that intended purpose provides a significant improvement compared to the existing device(s).
It may become evident, when doing initial searches, whether or not there are devices in the ARTG with the same technology, mechanism or approach. However, the critical issue when considering this criterion is whether or not there are devices in the ARTG with the same intended purpose (for the treatment, etc. of the condition) regardless of the technology, mechanism or approach involved (and regardless of whether the device is suitable for all or part of the patient population).
This means that unless it is clear that there are no devices included in the ARTG with the intended purpose of treating, etc., the particular condition, it is prudent to address the alternative criterion at (ii) in your application (relating to 'significant improvement').
Therefore, in your application, you should provide:
- if your view is that there are no devices with that intended purpose: a declaration to the effect that there are no medical device(s) of a kind included in the ARTG with the same intended purpose, along with a brief summary of searches completed to establish this
- otherwise: details of existing medical devices in the ARTG used in the monitoring, treatment, prevention or diagnosis of the condition, with a brief analysis of similarities and differences between your device and the existing device(s). Your analysis should address why the safety and performance of your device (when used for the intended purpose) provides a significant improvement compared to the existing device(s).
In addressing 'significant improvement' you may be able to draw on similar reasoning and facts as to why there is a 'major clinical advantage' (as required by criterion 3(i) and (ii): see further below).
Other factors you may wish to address in discussing 'significant improvement' include improved accessibility or acceptability.
Criterion 3: Major clinical advantage (or in the case of IVDs, major public health benefit)
For all medical devices (including IVDs) this criterion can be met where at least one of the following applies to the device:
- the new device is a breakthrough technology and there is evidence that it offers a major clinical advantage over existing technology
- there is evidence that the new device offers a major clinical advantage over existing alternatives included in the ARTG.
In addition, where the new device is an IVD, this criterion can be met where its early availability in Australia will result in a major public health benefit.
Major clinical advantage
'Major clinical advantage' is a term used in alternatives (i) and (ii) in criterion 3, which are discussed further below. A major clinical advantage should generally be understood as an improvement in the safety and/or performance of a medical device that is of a magnitude well beyond the minimum threshold of clinical significance.
A major clinical advantage with respect to the patient population may comprise, for example:
- improved performance for the population relevant to the intended purpose (for example, reduced hospitalisation, improved quality of life or better treatment effectiveness)
- a better safety profile for the population relevant to the intended purpose (for example, lower rates of mortality or adverse events).
Where a device treats an otherwise excluded patient population, then this may be considered to constitute a 'major clinical advantage' if the impact of this effect is considered to be sufficient when compared to existing technology or alternatives for that patient population.
In your application, you should provide a succinct summary of the available evidence with respect to:
- the magnitude of the demonstrated improvement in safety and/or performance
- the impact on patient outcomes taking into account both safety and performance
- comparison of safety and performance outcomes with the current standard of care and/or other medical devices or alternatives in the ARTG, as relevant.
Applicants are advised to present key data in tabular form to aid comparison.
Quality of evidence
Evidence will be assessed for quality (study type, size and design) and will also be considered in relation to issues involving comparability. The quality and nature of clinical evidence provided will be a significant consideration in determining whether sufficient evidence has been provided to demonstrate a major clinical advantage.
The following should be noted:
- single arm studies (and other study designs) with no comparator arm are generally considered inadequate evidence
- comparisons of datasets obtained through different methodologies (for example, a case series using the subject device with standard of care outcomes established from a literature search) are generally considered poor quality evidence and may be subject to greater scrutiny, as necessary, when assessing whether that data supports a major clinical advantage
- clinical advantages should generally be expressed in terms of person-centred outcomes, such as improvements in mortality or morbidity (though in some instances, a well-characterised biomarker may be acceptable)
- where study findings are expressed in terms of markers or intermediate measures of safety and performance, a clinically reasoned argument should be provided linking the study findings with the requirement of major clinical advantage
- although there is no rule regarding study size, those involving a sample size that is not statistically-powered will generally be considered poor quality evidence.
In describing the major clinical advantage, you should discuss results for all outcomes relevant to the device category or intended purpose, although emphasis can be placed on the outcome(s) demonstrating a major clinical advantage.
Where the context may involve both superior outcomes and inferior outcomes (or safety issues), this may affect the overall determination of whether there is a major clinical advantage. A balancing of benefit and risk will be required in considering whether any superior outcome is outweighed by other inferior outcomes or safety issues (and whether a major clinical advantage is demonstrated).
For further information, see Clinical evidence guidelines.
The case studies below set out particular scenarios and discuss the nature of the evidence provided.
Engineering or pre-clinical evidence is insufficient on its own; there should be sufficient clinical evidence to demonstrate a major clinical advantage (or public health benefit for IVDs).
Breakthrough technology offering a major clinical advantage over existing technology
This alternative (within criterion 3) relates to breakthrough technology. A breakthrough technology is a novel technology or novel application of an existing technology. Where there is evidence it offers a major clinical advantage over existing technology, that is, in the context of the monitoring, treatment, prevention or diagnosis of the relevant condition, then this criterion is met (noting only one of the alternatives in criterion 3 needs to be met).
In your application, you should provide a summary demonstrating novelty and advancement (in monitoring, treatment, prevention or diagnosis) associated with the use of your device.
The comparison with the existing technology should identify any relevant ARTG-listed devices. For breakthrough technology, comparison is generally made with the existing standard of care. Applicants should establish the existing standard of care with reference to the literature and current state of the art.
Major clinical advantage over existing alternatives included in the ARTG
This alternative (within criterion 3) requires the new device to be compared with existing alternatives included in the ARTG. An existing alternative does not necessarily have to be a device, it can be any therapeutic good included in the ARTG. There must be evidence that the new device offers a major clinical advantage over existing alternatives.
The existence of the major clinical advantage should be supported by sufficient clinical evidence.
In the case of IVDs: Early availability in Australia will result in a major public health benefit
This alternative (within criterion 3) relates only to in vitro diagnostic medical devices (IVDs). Note that it is possible that IVDs may also meet the other alternative requirements within criterion 3. Only one of the alternatives within criterion 3 must be met in order for criterion 3 to be satisfied.
Under this criterion, the decision-maker will assess whether the early availability in Australia of the IVD will result in a major public health benefit. In this context, the term 'public health' is generally understood as relating to the health of the Australian population.
In the normal course, the type of IVD device that may be expected to result in a major public health benefit (subject to the evidence of that benefit) is a device that provides a new diagnostic test or an improvement to an existing diagnostic test (see further below in relation to diagnostic tests).
In your application, you should provide evidence on how the availability of the new IVD will benefit the health of the Australian population and the extent of that benefit.
For example, where there is currently no diagnostic test, 'major public health benefit' should be demonstrated with reference to the literature and the state of the art. 'Major public health benefit' should be demonstrated through reference to sensitivity, specificity, positive and negative predictive values, likelihood ratios and the impact of the new test on health outcomes.
Further, where there is currently a diagnostic test, applicants should provide documentation of the improved sensitivity and specificity of the IVD compared to existing IVDs in the ARTG.
The patient population that will benefit from the improved diagnosis and/or treatment should be described. The evidence should be discussed with reference to:
- the particular characteristics of the condition (number of affected patients; transmission of the disease)
- the other available diagnostics
- methods of treating or preventing the identified condition.
In summary, you should provide a reasoned public health argument demonstrating an expected benefit to the health of the Australian population that incorporates device-specific evidence (including summaries of relevant studies) and public health evidence.
The application process
This part of the guidelines provides information on the application process for a priority applicant determination including the ability to have a pre-submission meeting. For more information refer to the assessment process and what happens once a decision on your application is made.
Pre-submission meeting
You may request a pre-submission meeting with us prior to submitting your application for a priority applicant determination. A pre-submission meeting seeks to provide non-binding guidance or feedback on the strength of the proposed application, and what to include in your application for a priority applicant determination. No guarantee is given at a pre-submission meeting as to whether a priority applicant determination would be approved.
To request a pre-submission meeting complete the Request for pre-submission meeting form. In the 'Subject matter for discussion' free text field of this form, indicate that the pre-submission request relates to an application for priority applicant determination for a medical device (or IVD).
Email your form to PriorityDevices@health.gov.au with the subject: Pre-submission meeting request - priority applicant determination for (name of medical device or IVD).
To maximise the benefit of a pre-submission meeting you should provide us with a briefing package at least one week prior to the meeting that includes:
- an agenda
- a draft of your application
- summaries of relevant supporting documents
- highlighted questions that you have for us.
Lodging an application
To apply for a priority applicant determination, you will need:
- a TGA client identification number and access to the business services portal (new sponsors will need to complete the organisation details form and submit to the TGA Business Services helpdesk using the address on the bottom of the form)
- a completed application for priority applicant determination form
- relevant supporting information listed below.
The application form and supporting information should be submitted via email to PriorityDevices@health.gov.au.
If your application is accepted for assessment, your invoice will follow shortly afterwards. Assessment of the application for a priority applicant determination cannot occur until you have paid the application fee.
Supporting information
Your application must be accompanied by written information in such detail as is reasonably necessary to allow the application to be properly considered. Supporting information can be provided as attachments, using the format outlined in our General dossier requirements.
Supporting information generally includes a document that addresses all three criteria plus any other relevant information, including summaries of:
- relevant background discussion and data
- clinical investigations (summaries of study reports or resultant peer reviewed articles) providing support with reference to the relevant criteria
- other important safety and performance data obtained in the preclinical and/or clinical setting.
The supporting information (separate from the application form) should ideally not exceed 20 pages.
The application, including attachments, should be concise. Do not include full clinical or technical reports.
Ensuring your application is complete
Please ensure your application for a priority applicant determination is complete. As we aim to assess applications within a relatively short time period (see timeframe details below), there is limited capacity for us to seek additional information and clarification from applicants. Uncertainties should be addressed at a pre-submission meeting.
The following additional details, if relevant, should be included in your application:
- whether you have submitted or are planning to submit, any other related applications regarding therapeutic goods
- confirmation that the intention is that an application for conformity assessment or ARTG inclusion will be submitted within 6 months of notification of a priority applicant determination having been made or confirmation of whether you have already made an application for conformity assessment or ARTG inclusion
- if you have regulatory approvals from other jurisdictions.
Fees
Once you have emailed us your application, you will be sent an invoice for the fee which must be paid before we begin assessing your application. Payments can be made via EFT, credit card (preferred) or cheque, and instructions on how to pay will be provided with the invoice.
For the current priority applicant determination fee for a conformity assessment (priority applicant) determination or for a medical devices (priority applicant) determination, see our Schedule of fees and charges.
Do not send payment before you receive an invoice from us.
We do not refund fees for applications that are withdrawn before a decision is made, or where the delegate refuses to make the priority applicant determination.
Withdrawing your application
You may withdraw your application for a priority applicant determination at any time before we make a decision on your application.
To withdraw the application, email PriorityDevices@health.gov.au and include:
- a statement that you wish to withdraw your priority applicant determination application
- the applicant's name
- the device name.
As noted above, withdrawn applications are not eligible for a refund of the priority applicant determination fee.
Special cases - including multiple devices and change applications
Multiple devices
Where an applicant seeks priority applicant determinations for more than one device in the same lineage (a predicate device) or family (similar device), this will generally require separate applications and the payment of separate application fees (full amounts).
Likewise, where an applicant seeks priority applicant determination for multiple devices that are designed to be used together for the same intended purpose, this may require separate applications to be submitted.
However, if the same clinical datasets provide the evidence base for multiple devices, then discussion with the TGA prior to submission is recommended to determine the optimal approach.
Change applications
If you propose making a change to extend the intended purpose of a device that is already included in the ARTG, and as a consequence, you need to submit a substantial change notification and application for your TGA-issued conformity assessment certificate, you may also apply for a priority applicant determination.
For example, this would be relevant to extending the intended purpose of a Class III, Class AIMD or Class 4 IVD medical device where the change constitutes a substantial change that requires approval by the TGA. The conformity assessment (priority applicant) determination application in this case would be assessed against the criteria with reference to the proposed new intended purpose.
Other scenarios
Other scenarios may arise where clarification around the applicability and requirements of the priority applicant determination process are needed.
The requirements specific to each case can be clarified with the TGA at the pre-submission meeting or via correspondence with PriorityDevices@health.gov.au.
The TGA assessment process
After you submit your application for priority applicant determination and pay the application fee, it will be assessed by a TGA delegate against the legislative criteria. The delegate will consider the information provided in your application and supporting documentation including the strength of the clinical evidence.
The assessment process is a streamlined one. As noted, once an application has been made, there is limited capacity to seek further information or clarification. Sponsors are strongly advised to ensure applications are complete and contain all required information. Gaps in the information or evidence provided may mean the delegate is not satisfied that the criteria are met. If criteria are not met, the application must be refused.
From time to time in the assessment process the TGA may seek advice from other sources including external advice.
Timeframe
There is no legislated timeframe for making a decision on an application for a priority applicant determination.
The TGA generally aims to assess applications within 20 working days (with day one being the first working day after the invoice is paid). Working days in this context excludes weekends and public holidays. The 20 working day period also excludes time taken to respond to any requests for additional information.
Requests for additional information
While you are encouraged to provide complete information in your application, we may require additional information or clarification from you during the assessment process.
Any request for additional information will include:
- the timeframe in which you should respond
- details of how to provide your response (including TGA contact details).
You will need to provide any additional information to us within the time specified. This is generally around 5-10 working days. Consistent with standard TGA processes, the TGA will 'stop the clock' on the expected 20 working day assessment period during the time taken for you to respond to a request for additional information.
If you do not provide a response within the time specified for responding (or within any extension of time agreed to by the TGA), the delegate will make a decision based on the information before them.
Notifying you of our decision
After the delegate has made a decision, you will be advised of the outcome as soon as practicable via a notification letter sent by email.
The notification letter will indicate whether the delegate has made, or refused to make, the priority applicant determination.
Where a determination has been made, the notification letter will include:
- relevant information about the determination, including specifying who and to what the determination relates
- the date on which the determination comes into force (the day of the notice), and the period that the determination remains in force (a 6-month period, subject to the Regulations - see below for more information on cessation of a priority applicant determination being in force).
If no application for conformity assessment, or ARTG inclusion, as relevant, has been made (including meeting application requirements) before the end of the 6 month period, the priority applicant determination ceases to be in force. This means any application for conformity assessment, or ARTG inclusion, as relevant, made after this period would not be entitled to priority consideration (unless you were successful in reapplying for a priority applicant determination).
Where the delegate refuses to make the determination, the notification letter will include the reasons for the decision (and details of the process for requesting a reconsideration of the decision).
Matters specified in the determination
Where a priority applicant determination is made the following information will be specified:
- the person who is the priority applicant
- the medical device to which the determination relates (in respect of design, material, physical characteristics, and a particular sponsor and manufacturer)
- the intended purpose of the device (consistent with the information in the application).
Subsequent (or concurrent) applications for conformity assessment or ARTG inclusion must correspond to the above and this information cannot be varied by an applicant.
The intended purpose is the proposed intended purpose for ARTG inclusion. This must be the same as the stated intended purpose at the time of the application for the priority applicant determination.
Publication of determination decisions
Where a priority applicant determination is made, the determination will be published on the TGA website.
We will not publish details of priority applicant determination applications that are refused or are withdrawn before a decision is made.
Steps once priority applicant determination is made
In many cases, a person will firstly make an application for a priority applicant determination and, once they have obtained a determination, then lodge the corresponding application for conformity assessment or ARTG inclusion, as relevant (within the 6 month period it remains in force, as referred to above).
However, it is possible that a person may already have an existing application for TGA conformity assessment or ARTG inclusion under review, and only apply for and/or obtain a priority applicant determination thereafter.
In either case, so long as the priority applicant determination is in force at the time the corresponding application is submitted or being processed (and the information specified in the determination about who and what the determination relates to matches the details in the corresponding application), the corresponding application will be given priority consideration.
Once a delegate makes a priority applicant determination, they will advise the relevant TGA section that this applies to any subsequent or concurrent corresponding application for conformity assessment or ARTG inclusion.
Where you make a subsequent application for conformity assessment or ARTG inclusion, you should highlight (in that application) that a priority applicant determination has been made. If you already have a pending application for conformity assessment or ARTG submission, you should highlight in your priority applicant determination application that this is the case.
Priority consideration process
The corresponding application for conformity assessment or ARTG inclusion will be assigned front-of-queue status through all TGA processes, including technical assessments and business processes (this is referred to as the 'priority pathway' in the flowchart of the priority applicant determination process below).
Whilst the legislated timeframes for these corresponding applications, where applicable, do not change, the general result is significantly reduced times for processing these applications. For example, there is a legislated timeframe of 255 working days for making a decision on a conformity assessment application where a design examination is required.
Information submitted as part of the application for a priority applicant determination does not automatically become part of any subsequent application for conformity assessment or ARTG inclusion. It is your responsibility to ensure that matters included in the application for a priority applicant determination are also included in your subsequent application for conformity assessment, or ARTG inclusion, where this is relevant to the criteria for those matters.
The criteria for conformity assessment, or for ARTG inclusion, are separate and different to the criteria for obtaining a priority applicant determination (and there will also be differences in processes).
For more information about applications for TGA conformity assessment or for ARTG inclusion see Standards, guidelines and publications (medical devices and IVDs).
Cessation, revocation or re-applying
Cessation of a priority applicant determination being in force
A priority applicant determination will automatically cease to be in force 6 months after the date that you are notified of the decision (to make the determination), if you have not submitted your application for conformity assessment or ARTG inclusion by this time (note that the application must also meet application requirements).
Where a priority applicant makes (or has already made) an application for conformity assessment or ARTG inclusion before the end of the 6 month period, the priority applicant determination remains in force during the processing of that application. However, the determination will cease to be in force where:
- the priority applicant withdraws the application for conformity assessment or ARTG inclusion
- the application for conformity assessment or ARTG inclusion lapses (in accordance with section 41EG or section 41FK of the Act, respectively)
- the application for conformity assessment or ARTG inclusion is finally determined.
Revocation of a priority applicant determination
The TGA has a discretionary power to revoke a priority applicant determination prior to the end of the 6 month period. This may occur if:
- you have not made an application for TGA conformity assessment or ARTG inclusion, or you have made such an application but it is not effective (it does not meet application requirements)
- the delegate is satisfied that the criteria specified for a priority applicant determination are no longer satisfied in relation to the medical device.
Generally, we will not routinely review priority applicant determinations while they remain in force with a view to considering whether a determination should be revoked.
Reapplying for a priority applicant determination
If you wish to reapply for a priority applicant determination after a determination has ceased, you will need to lodge a new priority applicant determination application.
We will assess the new application and the facts of the case as they exist at that time (against the criteria), noting it is possible that the facts may have changed since the original determination was made.
For new applications, you should provide the reference number for any relevant previous application for a priority applicant determination.
If a new priority applicant determination is made, the details of any corresponding application for conformity assessment or ARTG inclusion must match the details of the new priority applicant determination, for priority consideration to occur.
Case studies
The following case studies provide examples of possible scenarios and outcomes. Particular focus is given to the nature and type of evidence provided.
Case study 1
Bean's Devices Pty Ltd (Bean's) has developed Medical Device A that treats a common and seriously debilitating condition, using an established technology used for a novel purpose. The clinical evidence provided consists of an N=80 company-sponsored (not peer reviewed), single arm study, where the results were compared to benchmarks obtained from the literature regarding the current state of the art approach. Comparison of the datasets suggests that Device A achieves comparable performance, with a serious complication rate of 1-2% compared to 5% for the current state of the art treatment.
Outcome: A priority applicant determination was not made in relation to Device A because the applicant had not provided sufficient clinical evidence to support their claims against the criteria. The main issue was the poor quality of clinical evidence provided. The delegate assessed that the claim of a 'major clinical advantage' was marginal due to issues such as the insufficient magnitude of the effect and uncertain comparability of the datasets.
Case study 2
Bean's Devices Pty Ltd (Bean's) has developed Medical Device B, which uses improvements on existing technology to treat a relatively common but seriously debilitating condition. The clinical evidence provided consists of an N=400 Randomised Control Trial, published in a peer-reviewed journal. A summary of the study report indicates that the primary endpoint of all-cause mortality was significantly lower in those who received Device B compared to the major ARTG-listed alternative.
Outcome: A priority applicant determination was made in relation to Device B on the basis that sufficient clinical evidence (noting the high quality study design) had been provided to demonstrate a major clinical advantage.
Priority applicant determination process
The priority applicant determination process
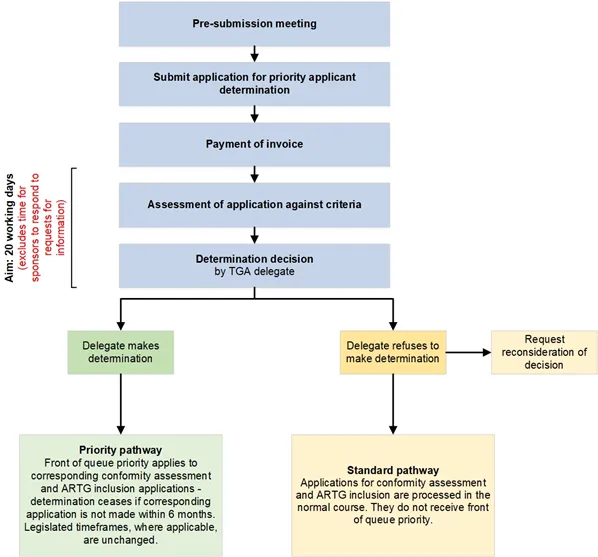
A flowchart detailing the process for priority applicant determination provided as a list with numbered steps.
Steps 3 to 5 are labelled with the text, "Aim: 20 working days (excludes time for sponsors to respond to requests for information)".
Step 5 includes various potential outcomes.
Flowchart steps:
- Pre-submission meeting
- Submit application for priority applicant determination
- Payment of invoice
- Assessment of application against criteria
- Determination decision by TGA delegate
- Delegate makes determination
- Priority pathway - front of queue priority applies to corresponding conformity assessment and ARTG inclusion applications - determination ceases if corresponding application is not made within 6 months. Legislated timeframes, where applicable, are unchanged.
- Delegate refuses to make determination
- Request reconsideration of decision
- Standard pathway - applications for conformity assessment and ARTG inclusion are processed in the normal course. They do not receive front of queue priority.
- Delegate makes determination
Page history
Title changed from 'Priority applicant guidelines for medical devices (including IVDs)' to 'Understanding priority applicant determination rules for medical devices including in-vitro diagnostics (IVDs)' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Updated title from Priority review designations medical devices (including IVDs) to Priority applicant guidelines for medical devices (including IVDs). Updated content, including flow chart, to be more accurate, detailed and consistent with current legislation and practices in consultation with Regulatory Legal Services Branch.
Addition of flowchart illustrating priority review process; update of application form details; update of details on lapsing/revocation of designations, addition of information for Criterion 1.
Application form provided online.
Original publication.
Title changed from 'Priority applicant guidelines for medical devices (including IVDs)' to 'Understanding priority applicant determination rules for medical devices including in-vitro diagnostics (IVDs)' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Updated title from Priority review designations medical devices (including IVDs) to Priority applicant guidelines for medical devices (including IVDs). Updated content, including flow chart, to be more accurate, detailed and consistent with current legislation and practices in consultation with Regulatory Legal Services Branch.
Addition of flowchart illustrating priority review process; update of application form details; update of details on lapsing/revocation of designations, addition of information for Criterion 1.
Application form provided online.
Original publication.

