UDI affects several existing TGA processes and activities. As a sponsor or manufacturer, it is your responsibility to understand where UDI impacts these processes.
Consent to Supply (CTS) process for UDI
If you cannot meet the UDI requirements after the mandatory compliance date for your medical device, you may choose to submit an application for consent to supply. You must lodge the application well in advance of the mandatory compliance date with supporting documentation. We must consider and make a decision on the application before you supply the non-compliant device.
We are establishing a simpler and lower impact CTS process for sponsors unable to comply with the UDI-related Essential Principles, reflecting the need to balance industry compliance with the UDI requirements with the safety risk if a device cannot be UDI compliant by the relevant UDI compliance start date.
Details on the streamlined process and commensurate fees for the UDI CTS will be published on the TGA website once finalised.
Market actions: Recalls, alerts and corrections
Where a UDI is available for a device, you must include it in any related:
- recalls
- market action customer letters.
When submitting a new market action notification through the TBS portal, enter UDIs in the applicable field on the ‘Product Report’ tab.
Where a UDI is not available, reports continue as per existing requirements and should include any applicable information.
Adverse events
Where a UDI is available, you must include it in adverse event reports. As the adverse event reporting system currently does not include a dedicated UDI data field, enter the UDI(s) as text in the report.
Patient Implant Cards
Manufacturers must provide Patient Implant Cards (PICs) for their implantable medical devices.
PICs must include:
- name of the device
- model of the device
- batch code, lot number or serial number of the device
- manufacturer’s name, address, and website.
For devices in scope of UDI requirements, the PIC must also include:
- the full UDI (UDI-DI and UDI-PI) in AIDC form
- the UDI-DI in HRI form.
PICs are not required to bear the UDI-PI in HRI form, as production information such as batch code, lot number or serial number is already required per existing PIC requirements.
Example of a UDI compliant PIC
Example of a UDI compliant PIC
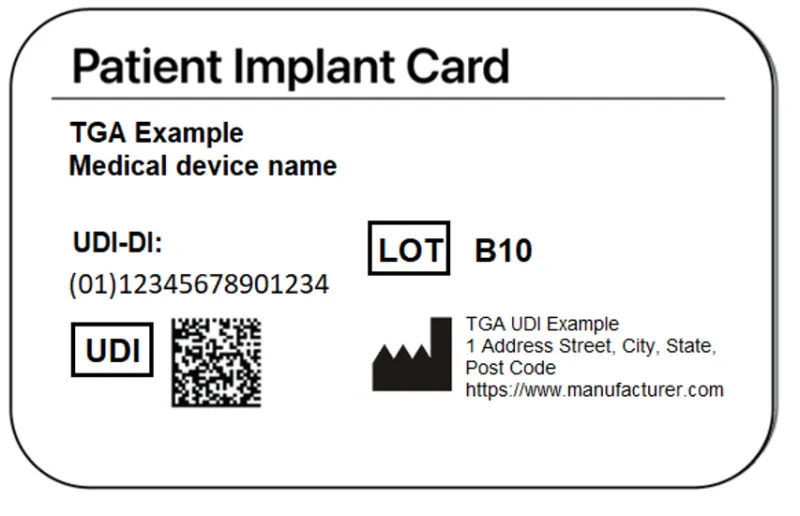
An example of a PIC displaying the UDI in AIDC and the UDI-DI in HRI.
If you are unable to meet the UDI requirements for Patient Implant Cards, you will need to seek Consent to Supply.
PICs for small non-sterile devices in Surgical Loan Kits (SLKs)
For small non-sterile devices in SLKs where only the UDI-DI is provided due to challenges associated with maintaining the UDI, only the UDI-DI is required on the PIC. The UDI-PI is not required.
Where the UDI-PI is not provided on the PIC for these devices, any market action must include all lots, batches or production runs of the device.
If the device is also supplied outside of the SLK, the PIC for the device supplied outside the SLK must have both the UDI-DI and the UDI-PI.
Patient Information Leaflets
You are not required to include the UDI on Patient Information Leaflets (PILs). However, you may choose to include the UDI voluntarily.
As a sponsor, you may choose to submit PILs to the AusUDID as either a:
- URL link
- PDF attachment.
If you include a PIL in your UDI record, you must keep it up to date.
Instructions for Use
You are not required to include the UDI on Instructions for Use (IFUs). However, you may choose to include the UDI voluntarily.
As a sponsor, you may choose to submit IFUs to the AusUDID as either a:
- URL link
- PDF attachment.
If you include an IFU in your UDI record, you must keep it up to date.
Certifications
You do not need to re-register or re-certify your medical devices when you amend your medical device labelling to meet the UDI requirements. This includes changes to:
- device labels
- Patient Implant Cards (PICs)
- any other supporting documents.
You are not required to provide evidence to the TGA that devices have changed to meet UDI requirements. However, you must submit a UDI record for each device to the AusUDID.
Audits
TGA audits
The TGA may review compliance with UDI requirements during:
- post-market reviews
- targeted audits
- routine inspections.
These audits may focus on whether sponsors have:
- submitted UDI records for all applicable devices
- maintained accurate and up to date data
- include UDI in required processes such as recalls.
As the UDI requirements form part of the Essential Principles, we may take regulatory action if you are non-compliant, including:
- suspension or cancellation of your devices from the Australian Register of Therapeutic Goods (ARTG)
- applying civil penalties as outlined in Part 4-11, Division 1 of the Act
- issuing infringement notices.
You can learn more on our website about compliance actions.
AusUDID reviews
The UDI Support Team will conduct periodic reviews of UDI records submitted to the AusUDID. These reviews will include checks for:
- Completeness – Mandatory and conditionally mandatory fields are populated
- Accuracy – The UDI data matches the ARTG inclusion and Issuing Agency rules
- Timeliness – Updates are made within the required timeframes
- Corrections – Reasons and descriptions for change are provided for corrections
- UDI Trigger rules – Changes to UDI Trigger data elements correctly result in a new UDI-DI and UDI record or are processed as a correction.
Review outcomes may include:
- requests for clarification or correction
- guidance on improving data quality
- escalation to TGA compliance teams, where required.
Record keeping requirements
As a sponsor, one of your existing ongoing responsibilities is to maintain distribution records for medical devices supplied in or exported from Australia. For details on existing distribution record requirements, see Distribution records.
UDI specific record keeping requirements
For UDI compliance, you are responsible for maintaining records of all UDIs used to identify devices that are in scope of UDI requirements:
- Sponsors must maintain records of the full UDI, including the UDI-DI and the UDI-PI to enable market actions at the model level or production level when necessary. This means recording each value, for example if there are 1,000 batch numbers, all 1,000 must be recorded.
- Sponsors must maintain a record of the types of production identifiers used (for example, batch number, lot number, serial number). This information forms part of the UDI record, so either the UDI record itself or the sponsor’s internal data either in your internal systems or prepared for AusUDID submission will satisfy this requirement.
- Sponsors must maintain a record of whether the device was directly marked, including whether the Direct Marking DI is the same or different from the Primary DI. Where the Direct Marking DI is different, the sponsor must maintain records of the Direct Marking DI itself. This information forms part of the UDI record, so either the UDI record itself or the sponsor’s internal data either in your internal systems or prepared for AusUDID submission will satisfy this requirement.
- If the UDI-PI includes production identifiers beyond those required for distribution records, for example serial number or software version number, the sponsor must maintain records of these. We do not prescribe how sponsors maintain these records.
We do not prescribe how sponsors hold this information. You may keep records of individual UDI-DIs and UDI-PIs, the full UDI or the individual elements that make up the UDI and related information in one or more system or records. Whether these are stored together or separately is subject to your systems and processes. The key requirement is that the full UDI and any related data can be retrieved when needed, such as during a market action.
For example: where components of the UDI-PI are already recorded under distribution record requirements, this is sufficient for UDI purposes. If the expiry date, lot, or batch numbers are already captured in distribution records, and you are able to use this information to identify specific devices when required, this will satisfy the record keeping requirement.
Retaining records of UDIs
As a sponsor, you must retain records for a minimum of 10 or 5 years, depending on the classification of your device.
| Classification of device | Record retention period |
|---|---|
| Class 4 IVDs | 10 years |
| Class III medical devices | 10 years |
| Class IIb implantable medical devices | 10 years |
| All other classifications | 5 years |
Example of UDI record keeping
Karolina the sponsor
Karolina supplies a Class III medical device. Her device has a UDI consisting of a UDI-DI and a UDI-PI with expiry date, batch number, and serial number.
Karolina can:
- maintain records of the full UDI, or
- maintain records of the UDI-DI and serial number and rely on existing distribution records for expiry date and batch number.
Karolina’s distribution records include expiry date and batch number but not the UDI-DI or serial number. She can:
- maintain records of the full UDI using her existing systems
- add the serial number and UDI-DI to her distribution records
- maintain each individual element in a way that suits her organisation.
As long as Karolina can retrieve the full UDI when needed, she meets UDI record keeping requirements. Because her device is Class III, she must keep these records for 10 years.
Fees and charges
There are no fees for submitting or maintaining UDI records in the AusUDID.
Ongoing management and maintenance of the AusUDID are included in annual charges.
Demonstrating compliance with UDI-related Essential Principles
Demonstrating compliance with the Australian UDI requirements will depend on the point in the device lifecycle and whether the device has an existing ARTG inclusion or is in a new Application for Inclusion.
Existing ARTG inclusions
For devices that are already included in the ARTG, you are not required to immediately or proactively demonstrate compliance. However, sponsors and manufacturers are expected to ensure their records, documentation and internal systems reflect the UDI requirements that apply in line with the compliance dates that apply to the specific device risk classification and type.
Essential Principle documentation
Your Essential Principles (EP) documentation should be updated to include any available information that demonstrates compliance with UDI related EPs. For example:
- EP13C.1 – A list of each device model and its associated UDI‑DI is sufficient.
- EP13C.2, EP13C.3 and EP13C.4 – A printout, screenshot, or link to the published UDI record in the AusUDID is acceptable.
- EP13C.5 (if applicable) – Procedures for direct marking or images of the directly marked device will satisfy this requirement.
These updates do not need to be made immediately but should be in place by the applicable mandatory UDI compliance dates for the device.
Quality Management System (QMS) updates
Your QMS should reflect the UDI‑related processes, decisions and actions taken, including:
- assignment and maintenance of UDI‑DIs
- management of UDI‑PIs
- labelling controls for UDI carriers
- direct marking procedures (if applicable)
- processes for UDI record updates in the AusUDID.
Compliance monitoring
Compliance with these obligations will be assessed through the TGA’s regulatory activities, including:
- post‑market reviews
- audits
- recalls and other market actions
- targeted compliance checks.
Please note that no separate or additional UDI‑specific audit program is in place and UDI compliance will be integrated into existing processes.
New ARTG inclusions
For new ARTG applications, assessment of UDI compliance will be incorporated into standard application review processes, including review of:
- product labels to ensure UDI Carrier requirements are met
- UDI‑DI assignment information
- direct marking evidence (if applicable)
- Patient implant cards (if applicable).
The TGA is currently finalising the UDI‑related compliance activities for new application processes. All information will be published on the TGA website once finalised.
Page history
- Renamed page from 'UDI and TGA processes' to 'UDI and other TGA processes or activities'
- Clarified requirements for Patient Implant Cards for small non-sterile devices supplied in surgical loan kits
- Clarified information on audits and AusUDID data reviews
- Added information on demonstrating compliance with UDI-related Essential Principles
Original publication.
- Renamed page from 'UDI and TGA processes' to 'UDI and other TGA processes or activities'
- Clarified requirements for Patient Implant Cards for small non-sterile devices supplied in surgical loan kits
- Clarified information on audits and AusUDID data reviews
- Added information on demonstrating compliance with UDI-related Essential Principles
Original publication.

