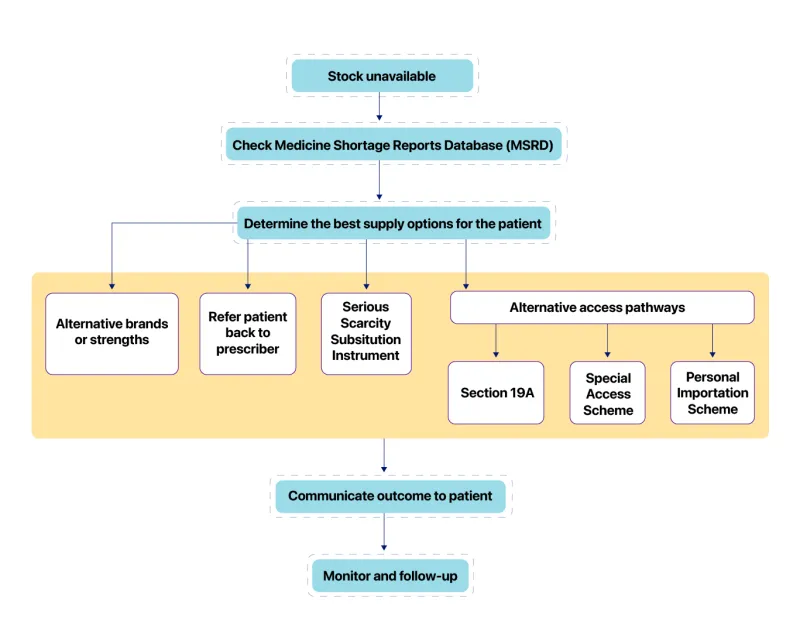
Helping pharmacists manage medicine shortages
Published
Related content
-
Medicine shortages - don't get caught short
Find out how mandatory reporting of medicine shortages will help you. -
TGA to permit conditional substitution to ease serious shortages
In response to the COVID-19 pandemic, the TGA is implementing Serious Shortage Medicine Substitution Notices -
What to know if you’re prescribed an unapproved therapeutic good
While we encourage health practitioners to prescribe approved therapeutic goods where possible, unapproved therapeutic goods can be accessed in limited circumstances.
In making these joint decisions to use an unapproved therapeutic good, your doctor is required to inform you of the potential benefits and risks.