Executive summary
During the COVID-19 pandemic, a rapid increase in demand for face masks resulted in a substantial increase in the number of these products included in the Australian Register of Therapeutic Goods (ARTG).
We received signals from various sources including frontline healthcare workers, the public, and State and Territory jurisdictions during the early stages of the COVID-19 pandemic on the quality and effectiveness of face masks available to them.
As part of the TGA's contributions to a national COVID-19 response, a post-market review (PMR) of all face masks included in the ARTG was conducted.
The objective of this review was to verify the sponsor's claims that face masks included in the ARTG conformed to claimed standards.
Note
This report relates to testing of disposable face masks and respirators products.
Throughout the report these are referred to collectively as ‘face masks' or ‘masks', except where distinction between the two product types is relevant.
Laboratory testing
For medical devices, sponsors are required to hold evidence or be able to provide evidence on request that demonstrates compliance with all applicable Essential Principles [1] as well as compliance with any standards claimed by the manufacturer of the device.
The laboratory testing in this review comprised of:
- Visual inspection
A visual examination of labelling, design, and construction quality. - Fluid resistance
Ability to provide a barrier against penetration by blood and/or other body fluids. - Particle Filtration Efficiency (PFE)
Ability to filter out small, aerosolised particles. - Sterility
Testing products that claim sterility to confirm they do not contain viable microorganisms.
Conclusion
The testing revealed, across all face mask products tested:
- 44% of products were found non-compliant for Visual Inspection
- 34% were found non-compliant for Fluid Resistance
- 26% were found non-compliant for PFE
- 15% were found non-compliant for Sterility
The post market review covered 2,276 ARTG entries.
During the review, 1,471 (~65%) of these entries were cancelled from the ARTG by the sponsor and 514 (~23%) were cancelled by the TGA.
The TGA laboratories tested 1,568 samples from 957 separate ARTG entries, comprising of over 250,000 individual specimens received.
Regulatory action taken by the TGA in response to testing and other aspects of the post-market review included: imposing Conditions of Inclusion (CoI) on ARTG entries; seeking variation to Global Medical Device Nomenclature (GMDN) codes; issuing product defect alerts; recalls; and cancelling entries from the ARTG.
The post-market review and testing of face mask products, along with subsequent regulatory actions, were effective in identifying and removing a significant number of non-compliant products from the ARTG.
This helped to ensure that the remaining products available for supply in Australia are suitable for their intended purposes.
Changes to the ARTG inclusion process for class I medical devices also helped reduce the risk of non-compliant products being supplied in the future.
Introduction
Face masks that are intended by their manufacturer to prevent the transmission of diseases between people or are intended to be used in a healthcare environment, are medical devices[2].
These devices are regulated by the TGA under the Therapeutic Goods Act 1989[3].
Unless exempt or excluded, medical devices must be included in the ARTG before being exported from, supplied, imported, or advertised in Australia.
Disposable face masks are used by frontline healthcare staff in settings where it is necessary to keep cross-contamination between the healthcare worker and the patient to a minimum.
The COVID-19 pandemic caused a rapid rise in demand for the manufacturing, importation and sale of face mask products.
The TGA received signals from various sources including from frontline healthcare workers, the public, and State and Territory jurisdictions during the early stages of the pandemic on the quality and effectiveness of these products available in Australia.
In response, and as part of the then Department of Health and Aged Care's contributions to a national COVID-19 response, the TGA undertook a post-market review of all face masks included in the ARTG.
The objective of the review was to verify whether these products met regulatory requirements and performed as intended, and in turn, inform the public of the safety and effectiveness of face masks available for supply.
This was achieved through a combination of desktop assessments, and laboratory testing.
The TGA's laboratory testing was targeted at a few key parameters, however, sponsors are required to hold evidence that demonstrates compliance with all applicable Essential Principles (EPs) as well as any standards claimed by the manufacturer of the device, including those referenced on the labelling or in the application for ARTG inclusion.
This report provides a summary of the testing and resultant regulatory action taken under the TGA's post-market review of face masks [4].
Device information
Before February 2020, there were a total of 102 face mask products included in the ARTG.
Between February 2020 and May 2020, during the initial stages of the COVID-19 pandemic, this number increased to 1,183 entries.
In total, 1,568 samples of 957 distinct ARTG entries were tested by the TGA Laboratories as part of this project.
Samples were obtained from a variety of sources including sponsors, retail stores, and from Commonwealth, State and Territory health agencies.
Test methods
Visual inspection
Visual Inspection involved a visual and physical examination of labelling, design, and construction quality to determine if the:
- Design of the device was consistent with the intended purpose and met the requirements set out by the manufacturer.
- Quality of the sample presented any notable defects or variability, and hence was compliant with the relevant provisions of the Essential Principles; and
- Goods complied with the labelling, packaging, and other requirements (including requirements relating to advertising) applicable to the goods.
Fluid resistance
Fluid resistance testing was conducted in accordance with the standard ISO 22609:2004(E), Clothing for protection against infectious agents – Medical Face Masks – Test method for resistance against penetration by synthetic blood (fixed volume, horizontally projected).
The principle of the test method is that a volume of synthetic blood is sprayed horizontally at a specimen mask through a small-diameter cannula.
The volume of fluid, distance to impact, cannula diameter and fluid velocity are predefined.
Claimed compliance levels of the masks (levels 1, 2, and 3), are tested using increasing pressure values (80, 120, and 160 mmHg) to increase the velocity of the blood spurt.
The inside of the face mask is checked visually (and with a swab when necessary) to see if any fluid has penetrated all the way through the face mask materials.
ISO 22609:2004(E) is referenced within the Australian Standard AS 4381:2015, Single-use face masks for use in health care, as a suitable test method to evaluate the resistance to penetration by synthetic blood.
Particle Filtration Efficiency (PFE)
PFE testing was performed to assess respirators for their ability to filter out small (<300 nm), aerosolised particles.
The mask is fixed stationary to a flow of aerosolised particles of set size distributions.
By measuring the number of particles in the aerosol stream before and after it passes through the respirator, the filtration efficiency is determined and reported as a percentage filtration efficiency value.
The filtration efficiency of tested respirator is compared against their claimed classification – e.g. N95 and KN95 respirators are required to have a filtration efficiency ≥95%.
This method was based on a modified version of the USA National Institute for Occupational Safety and Health's (NIOSH) published procedure TEB-APR-STP-0059 (STP-0059).
The standard testing procedure STP-0059 was designed to certify N95 respirators to confirm they meet the filter efficiency requirements set forth in United States 42 CFR, Part 84.
Sterility
Sterility testing is conducted to confirm whether a product is sterile, if the manufacturer has made that claim.
Sterility testing can be performed to detect any contamination arising from technical malfunction, an inadequate sterilisation process, human error or mix-ups between sterilised and non-sterilised goods.
Sterility testing of face masks was conducted in accordance with the principles of the harmonised British Pharmacopoeia (BP)/European Pharmacopoeia (EP)/US Pharmacopoeia (USP) Test for Sterility, as specified in the current BP Appendix XVIA.
The test method involves immersing the masks in a growth medium (broth) for a minimum of 14 days.
If after the incubation period there is no evidence of microbial growth detected, the sample complies with the Test for Sterility.
Results and discussion
Laboratory test results
Figure 1 shows the distribution of products tested alongside the percentage (%) of products in each category found to be non-compliant within an individual test.
A significant percentage of testing conducted resulted in non-compliant results, particularly for Visual Inspection and Fluid Resistance. 66% of respirators and 54% of surgical respirators failed Visual Inspection, for example.
Meanwhile, 49% of surgical respirators failed Fluid Resistance.
Figure 1: Percentage deficiency and number of samples tested by mask type and test type.
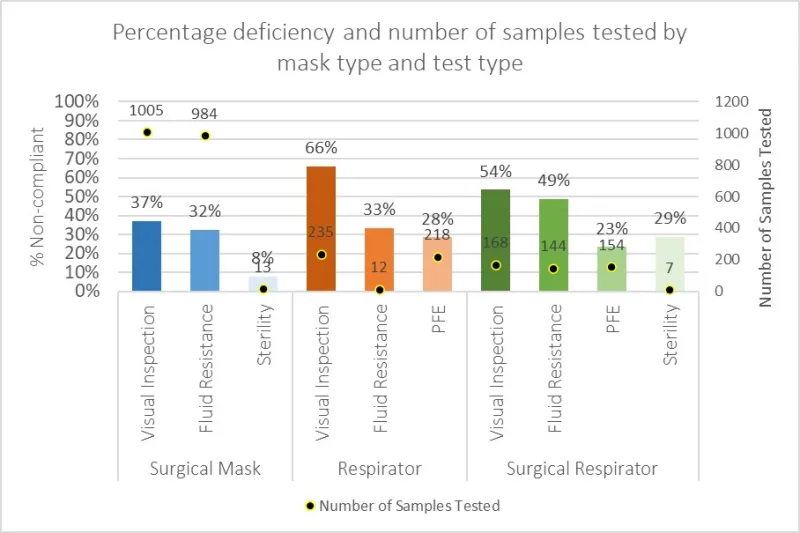
Table 1: Number of masks tested by mask type and test type
| Mask type and tests | Samples tested | % Non-compliant |
|---|---|---|
| Surgical mask | ||
| Visual inspection | 1005 | 37% |
| Fluid resistance | 984 | 32% |
| Sterility | 13 | 8% |
| Respirator | ||
| Visual inspection | 235 | 66% |
| Fluid resistance | 12 | 33% |
| PFE | 218 | 28% |
| Surgical respirator | ||
| Visual inspection | 168 | 54% |
| Fluid resistance | 144 | 49% |
| PFE | 154 | 23% |
| Sterility | 7 | 29% |
Table 1 shows a distribution of the product types tested and the tests conducted. More than half of all products tested were surgical face masks.
While almost all surgical face masks were tested for fluid resistance, only a small proportion of respirators were tested for fluid resistance as only a small proportion claimed to be fluid resistant.
Sterility testing was only conducted in cases where sterility was claimed by the mask manufacturer/sponsor and comprised 0.7% of all mask tests conducted.
For all product types, deficiencies by laboratory test type are shown in Table 2.
Across all products tested, 44% were found non-compliant for Visual Inspection, 34% were found non-compliant for Fluid Resistance, 26% were found non-compliant for PFE, and 15% were found non-compliant for Sterility.
Table 2: Percentage deficiency by test type
| Test type | Total number of samples non-compliant | Total number of samples tested | % Deficient |
|---|---|---|---|
| Visual inspection | 617 | 1408 | 44% |
| Fluid resistance | 393 | 1140 | 34% |
| PFE | 98 | 372 | 26% |
| Sterility | 3 | 20 | 15% |
Post-testing and regulatory action
As of 31 May 2023, the post market review had included the assessment of 2,276 ARTG entries.
During the review, 1,471 (~65%) of these entries were cancelled from the ARTG by the sponsor and 514 (~23%) were cancelled by the TGA (Table 3).
Disposable face masks products made up 92% (2,104 ARTG entries) of ARTG entries included in this review (other entries included various re-usable products), of which 957 ARTG entries (1568 samples) were received for laboratory testing.
Table 3: ARTG entries and cancellation outcomes from Post-Market Review, as of 31 May 2023
| Status | Number of ARTG entries |
|---|---|
| Included in the post-market review | 2,276 |
| Cancelled ARTG entries by the sponsor | 1,471 |
| Cancelled ARTG entries by the TGA | 514 |
| Remaining active ARTG entries | 291 |
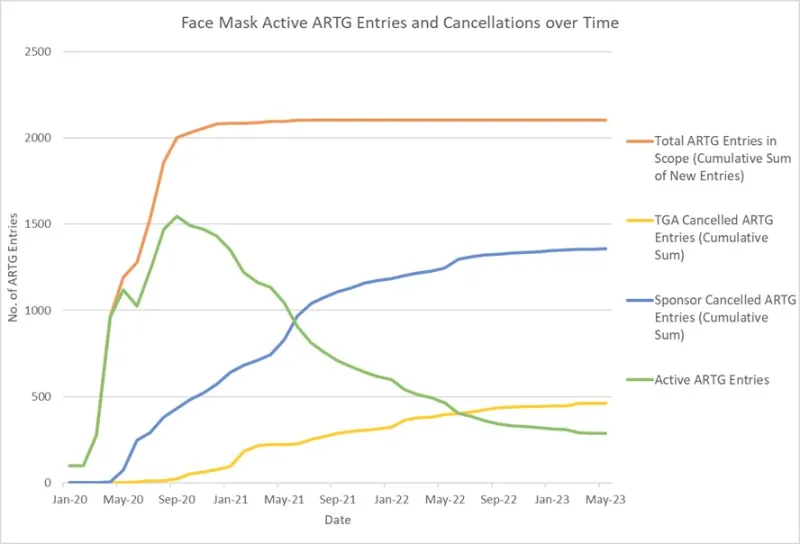
Figure 2 shows the number of active disposable face mask ARTG entries over the period encompassing the post-market review period. In this figure, the number of active ARTG entries is relatively low in January 2020.
A rapid increase in the number of active ARTG entries was observed up until late 2020, followed by a significant decrease in the years following, as the number of cancellations increased (both TGA cancellations and sponsor-initiated cancellations).
Figure 2: Active ARTG entries and cancellations over time of disposable Face Mask and Respirator products of relevant GMDN codes [5].
Other actions and procedural changes
In addition to ARTG cancellations[6], other forms of regulatory action taken by the TGA as a result of the review included:
ARTG entry corrections and related actions
- imposed Conditions of Inclusion (CoI),
- variations to amend GMDN[7] codes or intended purpose, and
Recall actions[8]
- product defect alerts,
- product notifications[9],
- and product defect corrections.
A total of 243 recall actions (product defect alerts, product notifications and product defect corrections) and 22 full recalls were issued as a result of the post-market review.
Changes to ARTG application requirements
Prior to the COVID-19 pandemic, applications to include face masks in the ARTG were processed in the same way as other Class I medical devices.
In October 2020, the TGA amended the inclusion process for all Class I medical devices to require a manufacturer's signed Declaration of Conformity to the Australian regulations to be attached to the application form, which is then checked before approval and inclusion of any Class I medical device in the ARTG.
This change was implemented due to the high levels of non-compliance seen early in the TGA's Facemask Post Market Review in both desktop reviews and laboratory testing results.
All face masks and respirator sponsors were required to provide evidence of the performance of the device (including test reports specifying sample sizes and target areas) to the applicable standards claimed[10].
References
- Quality, safety, and performance requirements for medical devices | Therapeutic Goods Administration (TGA)
- Therapeutic Goods (Medical Devices - Specified Articles) Instrument 2020, Schedule 1, Item 1. https://www.legislation.gov.au/Series/F2020L00463 .
- Therapeutic Goods Act 1989. https://www.legislation.gov.au/Series/C2004A03952 .
- Post-market review of face masks: Overview | Therapeutic Goods Administration (TGA)
- Global Medical Device Nomenclature (GMDN) terms included in figure: Antimicrobial surgical respirator (57792); Mask (12447); Mask, surgical, single use (35177); Public face mask, single-use (64821); Public respirator, single-use (57793); Surgical/medical respirator, single-use (57794).
- https://www.tga.gov.au/post-market-review-face-masks-cancelled-artg-entries
- Introduction to Global Medical Device Nomenclature (GMDN) | Therapeutic Goods Administration (TGA)
- https://www.tga.gov.au/how-we-regulate/monitoring-safety-and-shortages/manage-recall/about-australian-recall-actions
- https://www.tga.gov.au/resources/face-mask-non-compliance
- https://www.tga.gov.au/resources/resource/reference-material/evidence-requirements-face-masks-are-medical-devices
Page history
Version: V1.0
Description: Original publication
Author: Biomaterials and Engineering / TGA Laboratories
Effective date: 15 August 2025
Version: V1.0
Description: Original publication
Author: Biomaterials and Engineering / TGA Laboratories
Effective date: 15 August 2025

