Purpose
This page provides information for sponsors (product owners) and manufacturers of listed medicines and other parties involved in the medicine supply chain, such as ingredient suppliers.
Our intent is to raise awareness of the potential for nitrosamine impurities to form in certain listed medicine ingredients and formulated finished products containing such ingredients and provide information on how to assess the likelihood of susceptible ingredients forming nitrosamine impurities.
This advice aligns with the principles set out in the TGA publication Nitrosamine impurities in medicines but has been tailored to suit the unique environment of listed medicines.
For the purposes of this advice, the term ‘susceptible ingredient(s)’ refers to a discrete chemical entity (e.g. a vitamin) used in listed medicines that contains an amine functional group, as outlined in the section below, 'How nitrosamine impurities form.'
Notably, complex botanical ingredients (such as plant extracts) and ingredients derived from natural sources are excluded from this advice. These may be addressed in future publications concerning nitrosamine and nitroso impurities in listed medicines. This information will be updated as new information becomes available.
Listed medicines stakeholders should regularly visit this page and Nitrosamine impurities in medicines to remain current with developments.
What are nitrosamines
Nitrosamines are substances that can be found at low levels in various consumer products including treated water, processed meats, alcoholic beverages, vegetables, dairy products, cosmetics and air pollution.
Many nitrosamines are considered to be possible or probable human carcinogens. Long-term exposure to nitrosamines may increase an individual’s risk of developing cancer, with the risk dependent on the level and duration of exposure.
In medicines, nitrosamines are always considered to be impurities.
It’s important to understand that nitrosamine impurities can only form if the affected molecule contains an amine functional group. This specific group is essential for the chemical reaction that leads to the formation of nitrosamines, which may ultimately be present in the formulated finished product and potentially transfer to the consumer. If a molecule does not have an amine group, nitrosamine formation is not possible, and the measures outlined in this advice do not apply.
Obligations to mitigate nitrosamines in listed medicines
There is global recognition and concern regarding incidental nitrosamine impurities in medicines. To protect consumer safety, exposure to nitrosamines from avoidable sources, including listed medicines, should be minimised. Therefore, it is critical that sponsors ensure their products are free from, or contain minimal levels of, nitrosamine impurities.
Sponsors, and others involved in the supply chain of a medicine, should have measures in place that identify potential risk factors for nitrosamine formation and ensure that those risks are controlled.
Sponsors certify under 26A (for listed medicines) or under 26AB (for assessed listed medicines) of the Therapeutic Goods Act 1989, among other things, that their medicine is safe for the purposes for which it is to be used.
As such, sponsors have a regulatory obligation to mitigate the risk of nitrosamine impurities in their medicine. In general, sponsors are expected to maintain the quality of their products throughout the entire shelf life and review risk assessments when new regulatory developments arise.
How nitrosamine impurities form
Nitrosamines generally form by a reaction between nitrates or nitrites and certain amines in the medicine. The structures of nitrosamine impurities vary and depend on the base molecule from which they originate.
Under certain conditions, nitrosamine impurities may form during manufacture of ingredients containing a secondary (or tertiary) amine functional group(s) and/or manufacture of the dosage form containing these susceptible ingredient(s). Primary, secondary, tertiary amines, quaternary amines and amine oxides are all susceptible to nitrosamine formation.
Amines are classified based on the number of alkyl or aryl groups bonded directly to the nitrogen atom. A primary (1°) amine has one alkyl (or aryl) group attached to the nitrogen atom, a secondary (2°) amine has two, and a tertiary (3°) amine has three. The figure below refers, with alkyl or aryl groups denoted as R1 to R3. An illustration of a nitrosamine impurity formed from a secondary amine is presented in the righthand column.
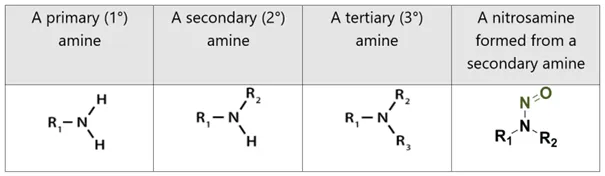
Note: Illustration of quaternary amines and amine oxides are beyond the scope of this publication.
Factors contributing to the formation of nitrosamine impurities depend on the type of amine functional group present. Secondary amines may form stable nitrosamine impurities and are therefore considered the highest threat.
Tertiary amines, while less reactive, can undergo dealkylation - losing an alkyl group from the nitrogen atom - which may also lead to nitrosamine formation. Other amine functionalities, such as primary amines are generally unstable and/or far less inclined to form stable nitrosamine entities.
Some risk factors for nitrosamine formation are provided below. More information is available at Nitrosamine impurities in medicines.
Risk factors for nitrosamine presence
Identified or plausible root causes of nitrosamine contamination in ingredients or formulated finished products can include:
- synthetic conditions (e.g. use of solvents during manufacturer that can directly or indirectly support nitrosation)
- carry-over/contamination with nitrosating agents and amines/amides
- contaminated recycled reagents, solvents and materials
- packaging (e.g. nitrite and nitrate sources such as nitrocellulose primary packaging)
- drying in/contact with atmospheric nitrous oxides
- degradation.
Listed medicine ingredient issues
We recognise that complex ingredients with variable composition - such as botanicals and other naturally derived medicinal substances - are not covered by this advice. Currently, limited data exists regarding the presence and extent of nitrosamine impurities originating from these types of ingredients.
While the possibility of nitrosamine formation cannot be ruled out, the absence of synthetic processing steps significantly reduces the likelihood of such impurities forming in susceptible ingredient(s). We may issue further advice on this as more information becomes available.
Listed medicine sponsor considerations
Sponsors of listed medicines, together with other parties involved in the supply chain, should be aware of the potential risk of nitrosamine contamination in listed medicines. They should also have measures in place to identify potential risk factors for nitrosamine formation and ensure that these risks are controlled.
Although nitrosamine impurities may form during the manufacture of the final dosage form or through contact with certain materials, the production processes for listed medicine dosage forms are typically not highly intensive.
Therefore, the most likely source of nitrosamines in the final product is the inclusion of susceptible ingredients capable of generating these impurities during their own processing.
To effectively control nitrosamine presence in the final product, risk mitigation should focus on the assessment and management of susceptible ingredients used in the formulation. Sponsors are strongly encouraged to collaborate closely with all parties in the supply chain - including raw material suppliers and manufacturers - to ensure that the risk of nitrosamine contamination is minimised through robust targeted assessment of any susceptible ingredients intended for use in the final medicine.
Nitrosamine risk assessments should be conducted for every susceptible (at risk) ingredient (including active and excipient ingredients), and, if necessary, the final formulated medicinal product. No set risk assessment is recommended. You should make all reasonable efforts to seek further information relevant to goods and conduct technically robust risk assessments, consistent with the requirements established in the quality system in operation.
Details and/or the outcome(s) of any risk assessments undertaken in this respect are not required to be submitted to us unless expressly requested.
It is important to note that the risk of nitrosation of a susceptible ingredient can vary depending on its source and the manufacturing process used to produce it. Confidence gained from a positive assessment of an ingredient obtained from one source cannot be automatically extended to the same ingredient from another source. Distinct supply chain sources of a susceptible ingredient should be independently assessed for potential nitrosamine impurities.
To support the design and implementation of nitrosamine risk assessments, the following contributing factors are provided for consideration in the design and implementation of any nitrosamine risk assessment undertaken.
Importantly, given the dynamic nature of this issue, this is not an exhaustive or definitive list. This information should be taken only as a guide.
| Does the ingredient under review have the following potential nitrosamine risk factors? | Yes | No |
|---|---|---|
| 1. Does the ingredient contain a secondary or tertiary amine functional group(s)? | ||
| 2. Do any known impurities, reagents or residual solvents contain a secondary or tertiary amine functional group? | ||
| 3. Are secondary or tertiary amines used in the ingredient synthesis (including the manufacture of starting materials/precursors/intermediates)? | ||
| 4. Could amines or amine sources be present as impurities in starting materials, reagents, catalysts/processing aids or solvents (e.g. dimethylformamide)? | ||
| 5. Is inorganic or organic nitrite or nitrate used in the ingredient synthesis (including in the manufacture of starting materials/intermediates)? | ||
| 6. Are potential nitrite or nitrate sources present in the ingredient synthesis (including in the manufacture of starting materials/intermediates)? | ||
| 7. Could impurities with nitrites or nitrite sources be present in the starting materials, reagents, catalysts/processing aids or solvents? For example, nitrates + reducing agents, HNO3 + reducing metals, urea/ammonium + hypochlorite/chlorine. | ||
| 8. Could nitrosamines be present as impurities in the starting materials, reagents, catalysts, processing aids or solvents? | ||
| 9. Is the ingredient dried in/or widely exposed to environmental air during manufacture? For example, fluid bed drying, or spray drying at elevated temperatures using environmental air. | ||
| 10. Are nitrite sources present or potentially present in the manufacturing process of the ingredient? For example, nitrates + reducing agents, HNO3 + reducing metals, urea/ammonium + hypochlorite/chlorine, solvents containing a secondary or tertiary amine functional group. |
| Consideration points applying to the final medicine | Yes | No |
|---|---|---|
| 11. Does manufacture involve drying with contact with untreated environmental atmosphere? For example, fluid bed drying, or spray drying at elevated temperatures using untreated air which may contain elevated nitrous oxides. | ||
| 12. Are there any other potential sources of nitrosamine present in the manufacture of the medicine? E.g. the potential presence of nitrosamine impurities in processing aids, or recycled solvents supplied by third parties should be carefully considered. |
Cont. 2
If the answer to any of the points above is ‘yes’, or you are aware of any other factors that may predispose the ingredient or finished formulated medicine to nitrosamine impurity formation, then the potential presence of nitrosamine impurities should be investigated.
If a significant level of nitrosamine impurity is identified or reasonably anticipated, appropriate corrective and preventive actions (CAPAs) should be implemented. This may include, but not limited to, discussions with manufacturers to review manufacturing processes/steps that increase the risk of nitrosamine formation and take corrective steps to mitigate the risk.
Reference is made to the TGA’s published information on acceptable intake (AI) limits for nitrosamine structural types and other nitroso impurities, which provides a framework for calculating the limit.
This information must be considered when assessing the overall risk associated with the use of any ingredient for use in a listed medicine - for which a credible nitrosamine impurity presence has been established or is probable.
Crucially, when evaluating nitrosamine risk in a formulated listed medicine, the total contribution of nitrosamine impurities from all sources - including both active and excipient ingredients - needs to be accounted for. The presence of a nitrosamine impurity above the acceptable limit indicates that the safety and quality of the medicine may be unacceptable.
Other considerations
Nitrosamines are one class of nitroso-compounds that can form under nitrosating conditions. Other nitroso-structures, such as N-nitrosoguanidines, N-nitroso-indoles, and N-nitrosoureas, may also form under similar conditions. Additional information on these compounds is available on our website.
When assessing the potential formation of nitrosamine impurities, the possible presence of other nitroso-impurities - beyond nitrosamines - should also be considered.
Note that information is available on our website on Acceptable Intake (AI) limits for nitrosamines and other nitroso-structures including N-nitrosoguanidines, N-nitroso-indoles and N-nitrosoureas.
Further information
For questions regarding listed medicines, email nonprescriptionmedicines@health.gov.au
Useful resources
- PIC/S Guide to GMP for medicinal products
- Regulatory Experiences with Root Causes and Risk Factors for Nitrosamine Impurities in Pharmaceuticals
- Health Sciences Agency (HSA) Singapore Appendix 2: Assessment aid for assessing nitrosamine risk
- Technical guidance document on minimising and determining nitrosamines in cosmetics
Page history
- Title change from 'Understanding nitrosamine risk in listed medicines' to "Nitrosamine risk in listed medicines'
- Title change from 'Understanding nitrosamine risk in listed medicines' to "Nitrosamine risk in listed medicines'

