Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is for medical practitioners, Human Research Ethics Committees (HRECs), specialist colleges and sponsors of 'unapproved' therapeutic goods and provides information about the:
- Authorised Prescriber Scheme
- roles and responsibilities of HRECs, specialist colleges, Authorised Prescribers, sponsors and TGA in relation to the Authorised Prescriber scheme
- legal basis for supply of 'unapproved' therapeutic goods
This guidance also provides details about relevant amendments to the Therapeutic Goods Regulations 1990 to streamline the application process for medicines considered to have an established history of use.
This document replaces previous guidance on this topic.
This information is provided for guidance only and should not be relied on to address every aspect of the relevant legislation.
You should seek your own independent legal advice to ensure that all of the legislative requirements are met.
Legislation
Authorised Prescriber Scheme
Generally, therapeutic goods must be included in the Australian Register of Therapeutic Goods (ARTG) before they can be imported into, supplied in, and exported from Australia. Therapeutic goods that are not included in the ARTG (described by us as 'unapproved' goods) cannot be lawfully supplied in Australia unless exempt, approved or authorised under the therapeutic goods legislation. Unapproved therapeutic goods have not been assessed by the TGA for quality, safety or efficacy.
The therapeutic goods legislation provides a number of avenues that allow access to therapeutic goods that are not included in the ARTG. The Authorised Prescriber (AP) scheme allows authorised medical practitioners to access and supply a specified ’unapproved’ therapeutic good (or class of ‘unapproved’ therapeutic goods) to a class of patients with a particular medical condition.
An Authorised Prescriber has authority to access the ‘unapproved’ therapeutic good directly for patients in their immediate care without requiring separate approval for individual patients. The named medical practitioner on the TGA AP authority letter is the only practitioner authorised to access the unapproved therapeutic good using the specific approval letter.
Who can become an Authorised Prescriber
Only medical practitioners can apply to become Authorised Prescribers under the Therapeutic Goods Act 1989. The therapeutic goods legislation defines a medical practitioner as ‘a person who is registered, in a state or internal territory, as a medical practitioner’. In addition, the HREC (if applicable) and TGA Delegate must be assured that the medical practitioner has the qualifications and experience necessary to appropriately manage the medical condition and use the ‘unapproved’ therapeutic good.
To become an Authorised Prescriber, applicants must:
- be a medical practitioner with general registration, including limited registration, or specialist registration with the Australian Health Practitioner Regulation Agency (AHPRA)
- have the training and expertise appropriate for the condition being treated and the proposed use of the ‘unapproved’ therapeutic good
- be able to best determine the needs of the patient
- be able to monitor the outcome of therapy.
Note:
- Only specialist medical practitioners are eligible to become Authorised Prescribers for Category 2, 3, 4 and/or 5 medicinal cannabis products for their patients under the age of 18 years (paediatric patients)
- Only specialist medical practitioners are eligible to become Authorised Prescribers for unapproved medical devices
- Only Psychiatrists are eligible to apply to become Authorised Prescribers for psychedelics (MDMA and psilocybine)
- Medical practitioners with provisional registration are not eligible to become an Authorised Prescriber, but may be able to access ‘unapproved’ therapeutic goods for individual patients under the Special Access Scheme rules
- Medical practitioners with non-practising or student registration are not eligible to become an Authorised Prescriber
- Other health practitioners, including dentists and nurse practitioners, are not eligible to become Authorised Prescribers. These practitioners may be able to access ‘unapproved’ therapeutic goods for individual patients under the Special Access Scheme rules.
Roles and responsibilities
The TGA
We administer the Therapeutic Goods Act 1989 (the Act), the Therapeutic Goods Regulations 1990 (the Regulations), and various Orders and Determinations, and regulate the quality, safety and efficacy of therapeutic goods as well as access to therapeutic goods that have not been approved for general use (‘unapproved’ therapeutic goods).
The TGA:
- encourages the use of products included in the ARTG
- determines whether there are emerging safety concerns that would make approval or endorsement inappropriate
- determines whether the requirements for authorisation as an Authorised Prescriber have been met.
Medical practitioners
Medical practitioners who wish to apply to become Authorised Prescribers must:
- determine whether any suitable alternative therapeutic goods are included in the ARTG
- apply for approval from an HREC or endorsement from a specialist college (if applicable)
- submit an application to us.
Medical practitioners who become Authorised Prescribers must:
- remain informed about changes to the benefits and risks of the ‘unapproved’ therapeutic good as they arise
- consider the potential benefits and risks of the ‘unapproved’ therapeutic good for each patient it is accessed for
- obtain written informed consent from each patient before prescribing
- ensure supply of the goods is available through or pharmacy
- monitor the patient during and after use of the ‘unapproved’ therapeutic good
- provide the TGA with a six monthly report for the periods 1 January to 30 June and 1 July to 31 December. These reports are a requirement under the Therapeutic Goods Regulations (1990), section 47B and must be supplied to us within one calendar month after the reporting period ends
- inform us of adverse events associated with use of the good
- meet any conditions the TGA, HREC or specialist college applies to the approval or endorsement
- comply with relevant state or territory legislation governing the supply of therapeutic goods. Approval as an Authorised Prescriber does not override state or territory legislation
- inform us if the AP authority if no longer required.
HRECs and specialist colleges
HRECs and specialist colleges:
- evaluate a medical practitioner’s submission (where applicable) and, if appropriate, approve or endorse it
- if the application is approved or endorsed, provide the medical practitioner with a letter declaring they have reviewed all necessary documentation and clearly stating this approval or endorsement
- ensure the approval or endorsement defines if paediatric patients are included, in addition to adult patients
- monitor the medical practitioner’s use of the ‘unapproved’ therapeutic goods to ensure continued endorsement is appropriate. Examples of monitoring that have been undertaken by HRECs and specialist college have included the requirement for medical practitioner to submit to them:
- reports outlining the number of patients who have been treated
- adverse event or product defect reports
- consider any new information available to determine whether it would be appropriate to continue the endorsement or approval.
Sponsors of ‘unapproved’ therapeutic goods
- must obtain a copy of the TGA authority letter from the medical practitioner before supplying the ‘unapproved’ therapeutic good to the medical practitioner
- supply the ‘unapproved’ therapeutic good, at their discretion
- monitor the use of the unapproved therapeutic good, report adverse events and product defects and record the balance of benefits and risks
- provide TGA with six monthly reports on the supply of ‘unapproved’ goods1. reports are a requirement under the Therapeutic Goods Regulations (1990), section 47B
- inform us of emerging safety concerns associated with the use of ‘unapproved’ therapeutic goods that they supply.
How to become an Authorised Prescriber
There are 2 pathways medical practitioners may use to apply to become an Authorised Prescriber, depending on the unapproved therapeutic good to be prescribed:
- established history of use pathway
- standard pathway.
Established history of use pathway
If the medicine, concentration (if any), dosage from, route of administration and indication match an entry in subregulation 12B(1B) or 12B(1C) of the Therapeutic Goods Regulations 1990, then HREC approval or specialist college endorsement is not required by the TGA. See the list of medicines with an established history of use.
HREC or institutional approval may still be required to use certain ‘unapproved’ therapeutic goods within an institution such as a hospital. Medical practitioners need to liaise with the relevant institution to confirm the institutions requirements.
To become an Authorised Prescriber using the ‘established history of use pathway’, a medical practitioner should follow these steps:
- check if the ‘unapproved’ therapeutic good is included in the list of medicines with an established history of use (if not, refer to the standard pathway on this page)
- complete an online application using the SAS & Authorised Prescriber Online System to the TGA
- the following details are required:
- medical practitioners name and contact details
- ‘unapproved’ therapeutic good details and indication or item code.
- the following details are required:
Medical practitioners have access to a dashboard on their online system account where they can:
- track the status of their application
- search for previously submitted applications using parameters such as product, submission date and status (i.e. approved, rejected, withdrawn, completed)
- download a PDF copy of the application receipt
- identify applications that are expiring or that have expired
- download a copy of the TGA decision letter
- clone (copy) previously submitted AP submissions
- submit their AP six-monthly report figures.
Further information on using the online system can be found in the Special Access Scheme & Authorised Prescriber Scheme Online system guidance document.
- The TGA will assess the application and provide further correspondence once reviewed.
To renew an application, an Authorised Prescriber will need to follow the above steps and ensure that all six monthly report figures for the previous authority have been submitted via their online system account.
Standard pathway
To become an Authorised Prescriber of therapeutic goods that are not included in subregulation 12B(1B) or 12B(1C) of the Therapeutic Goods Regulations 1990, a medical practitioner should follow the ‘Standard pathway’ steps:
- Submit an application to an HREC or a specialist college as outlined in the Applying for HREC approval or specialist college endorsement section on this page and receive approval.
- Complete an online application using the SAS & Authorised Prescriber Online System to the TGA.
The online system allows medical practitioners to access a dashboard where they can:
- track the status of their application
- search for previously submitted applications using parameters such as product, submission date and status (i.e. approved, rejected, withdrawn, completed)
- download a PDF copy of the application receipt
- identify applications that are expiring or that have expired
- download a copy of the TGA decision letter
- clone (copy) previously submitted AP submissions
- submit their AP six monthly report figures.
Further information on using the online portal system can be found in the Special Access Scheme & Authorised Prescriber Scheme Online system guidance document.
- Attach the HREC letter of approval or a letter of endorsement from a specialist college (where required), including a declaration that all necessary documentation has been reviewed
- The TGA will assess the application and provide further correspondence once reviewed.
To renew an application, an Authorised Prescriber will need to follow the steps above and ensure that all six monthly report figures for the previous authority have been submitted via their online system account.
Where to find a HREC or specialist college
HRECs
In the therapeutic goods legislation, an ethics committee means a committee:
- constituted and operating as an ethics committee in accordance with guidelines issued by the CEO of the National Health and Medical Research Council (NHMRC) as in force from time to time, and
- which has notified its existence to the Australian Health Ethics Committee (AHEC) established under the National Health and Medical Research Council Act 1992.
A list of registered ethics committees is available on the NHMRC website.
Specialist colleges
For ‘unapproved’ medicines or biologicals – if the medical practitioner is engaged in clinical practice outside a hospital and does not have access to an ethics committee, then the medical practitioner may obtain endorsement from a specialist college that has expertise relevant to the treatment of the medical condition for which authority is sought.
For ‘unapproved’ medical devices – if the medical practitioner does not have access to an ethics committee that:
- has the expertise relating to the use of the ‘unapproved’ good or
- conducts its activities in the geographical area where the approval is sought
then the medical practitioner may seek endorsement from a specialist college that has expertise relevant to the treatment of the medical condition for which the authority is sought.
The therapeutic goods legislation defines a ‘specialist’ as having the same meaning as in the Health Insurance Act 1973. This includes that the medical practitioner is a fellow of a ‘relevant organisation’ in relation to the specialty.
A list of these ‘relevant organisations’ are provided in Schedule 1 of the Health Insurance Regulations 2018. Therefore, the list of ‘relevant organisations’ are taken to be the ‘specialist colleges’ referred to for the purposes of the Authorised Prescriber scheme. Medical practitioners may only obtain specialist college endorsement from one of the ‘relevant organisations’.
Endorsement from a specialist society or other expert body cannot be accepted by the TGA. However, specialist colleges may choose seek advice from an expert body or specialist society when providing endorsement.
Applying for HREC approval or specialist college endorsement
The TGA does not provide a set template for HREC or specialist college applications. The medical practitioner’s application for HREC approval or specialist college endorsement (if required) must be made in writing and provide sufficient evidence to justify the use of the ‘unapproved’ therapeutic good. An application for HREC or specialist college approval or endorsement must contain details of the:
- medical practitioner applying for Authorised Prescriber status
- ‘unapproved’ therapeutic good
- patient group to be treated (paediatric/adult)
- clinical justification for the use of the ‘unapproved’ therapeutic good.
Medical practitioner details
The medical practitioner’s details to include are:
- name
- contact details (postal address, phone number, fax number and email)
- details of their qualifications, specialty, training and experience
- generally, applications from medical practitioners with non-practising, student, provisional registration (requiring supervised practice), or conditions placed on their registration will not be considered for the Authorised Prescriber scheme
- have the training and expertise appropriate for the condition being treated and/or the proposed use of the ‘unapproved’ therapeutic good
- a description of how they propose to use the ‘unapproved’ therapeutic good
- details of the site(s) at which the ‘unapproved’ therapeutic goods will be used.
The application should also provide evidence that the medical practitioner has:
- the qualifications and experience necessary to appropriately manage the medical condition, patient group and use the ‘unapproved’ therapeutic good
- access to the facilities needed to appropriately administer and monitor treatment.
Note:
- medical practitioners with provisional registration are not eligible to become an Authorised Prescriber, but may be able to access ‘unapproved’ therapeutic goods for individual patients under the Special Access Scheme rules,
- non-practising and student registration types are not eligible to become an Authorised Prescriber.
Generally, medical practitioners will have to demonstrate a higher level of experience and training to be approved as Authorised Prescribers of therapeutic goods that:
- are indicated for highly specialised medical conditions
- have significant safety risks
- require specialised monitoring
- require specialised administration or handling.
‘Unapproved’ therapeutic good description and evidence
The application should contain evidence of the ‘unapproved’ therapeutic good’s suitability for the intended indication that supports the clinical justification the medical practitioner has provided.
The application should include the following details of the ‘unapproved’ therapeutic good.
Description
For ‘unapproved’ medicines:
- active ingredient
- strength/concentration
- dosage form
- sponsor
- whether the good is approved for the indication by an overseas regulatory body.
For ‘unapproved’ biologicals:
- name of biological
- sponsor
- whether the good is approved for the indication by an overseas regulatory body.
For ‘unapproved’ medical devices:
- name of the medical device
- sponsor
- whether the good is approved for this indication by an overseas regulatory body.
Use and monitoring
The application should detail:
- the dosage range (where applicable)
- the route of administration or type of sample for IVDs
- the duration of treatment
- how the medical practitioner will determine if the use is effective
- how the medical practitioner will determine whether an adverse event has occurred
- what monitoring is required, how it will be done, and the interval and duration of monitoring.
Efficacy and safety
The application must contain information on:
- the ‘unapproved’ therapeutic good’s efficacy and expected benefits
- any known/expected adverse effects, risks and safety issues
- related toxicology.
Evidence
The application should contain appropriate sources of evidence to support the use of the ‘unapproved’ therapeutic good. The sources of evidence for data, with the highest level of significance first, in decreasing order are:
- ‘unapproved’ therapeutic good information documents (of equivalent) (if the good is approved by an overseas regulator)
- randomised controlled trials
- non-randomised controlled trials
- individual case studies
- consensus opinion of specialist colleges and societies.
Less serious conditions require stronger evidence than more serious medical conditions.
Figure 1: Evidence requirements and the seriousness of the medical condition
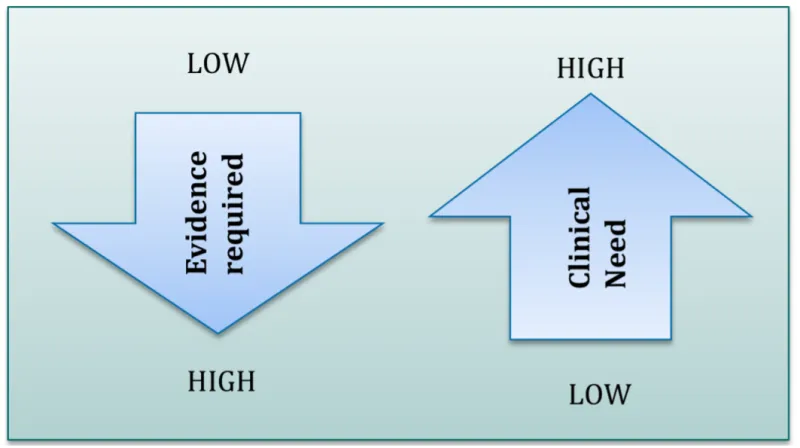
Diagram shows inverse relationship between evidence required and clinical need.
A downward-pointing arrow labelled 'Evidence required' ranges from 'LOW' at the top to 'HIGH' at the bottom.
An upward-pointing arrow labelled 'Clinical Need' ranges from 'LOW' at the bottom to 'HIGH' at the top.
Global regulatory status
The global regulatory status of the ‘unapproved’ therapeutic good may affect the level of evidence required in the application.
This table describes differences in global regulatory status and the effect that status may have on the level of evidence required.
The information in Table 1 is provided as a guide only.
| Regulatory status | Possible effect on the level of extra evidence required to be submitted to a HREC or specialist college |
|---|---|
| Goods which are not approved in Australia, but are approved for the indication and the conditions of use in countries with a regulatory standard comparable to Australia | Decreased |
| Goods previously approved by the TGA which have been withdrawn for non-safety reasons | Decreased |
| Goods which are not approved in Australia, but are approved in countries with regulatory standards that are not comparable to Australia | Increased |
| Goods that have not been approved anywhere for the indication and are still undergoing clinical trials | Increased |
| Goods previously approved by the TGA which have been withdrawn for safety reasons | Increased |
| Goods that have not been granted registration in Australian for safety reasons | Increased |
When an HREC or specialist college assesses your application, they should consider the following factors to determine what level of evidence is required:
- whether other treatments registered on the ARTG are available and suitable for the intended class of patients
- the seriousness of the medical condition
- the global regulatory status of the therapeutic good
- the relevant experience and qualifications of the applicant.
You may wish to contact the HREC or specialist college before you submit your application to ensure you submit the necessary evidence.
Clinical justification for the use of the unapproved therapeutic goods
We encourage the use of approved therapeutic goods as these have been assessed for safety, quality and efficacy. The clinical justification for use of an ‘unapproved’ therapeutic good should provide sufficient evidence to demonstrate that this use is appropriate, considering the availability of any approved goods that may be suitable alternatives.
The clinical justification should contain information on the:
- indication for which the unapproved therapeutic good will be used
- seriousness of the condition
- expected benefits of the proposed treatment versus its potential risks.
It should also address the circumstances where there are approved treatments for the same indication, specifically:
- have they been attempted or used?
- will they be attempted prior to supplying the ‘unapproved’ therapeutic good?
- why are they inappropriate?
- why is the proposed ‘unapproved’ therapeutic good a more appropriate option than any approved available alternative?
- how the risk associated with the use of an ‘unapproved’ therapeutic good will be managed
- the monitoring that will be undertaken
- the process of investigating and reporting adverse events.
The following are not acceptable justifications for the use of an ‘unapproved’ therapeutic good:
- that the ‘unapproved’ therapeutic good is less expensive than any suitable approved treatment
- personal preference for an ‘unapproved’ therapeutic good.
TGA decision
Applications that we authorise
If we approve your application, we will send you an approval letter. The letter will state the:
- approved ‘unapproved’ therapeutic goods and dosage form
- approved class of patients for a particular indication
- requirements for reporting
- any conditions applied to the approval
- the duration of approval.
All approvals are subject to general conditions. We may also apply specific conditions on a case-by-case basis. You must meet these conditions to retain your approval. The approving HREC or endorsing specialist college may also apply conditions to your approval.
Once you have been authorised to be an Authorised Prescriber of an ‘unapproved’ therapeutic good, you may prescribe that good to patients in your care.
Applications that we do not authorise
If we do not authorise your application, we will send you a letter stating:
- that an authorisation has not been granted
- the reasons for the decision
- the contact details of the delegate who made the decision, if you wish to discuss the decision
- the process if you want to appeal the decision.
You should inform the HREC or specialist college (if applicable) which approved or endorsed your application of our response and provide them with a copy of the decision letter.
If we do not authorise your application to become an Authorised Prescriber, you may submit a new application addressing the reasons for rejection.
Appeals mechanism
Informal appeals
If you wish to appeal a TGA decision, and before you make a formal appeal, you should contact the delegate who evaluated your submission to discuss the matter informally.
Formal appeals
If you disagree with the outcome of an informal appeal, you can make a formal appeal:
You must have attempted to appeal a decision under section 60 of the Therapeutic Goods Act 1989 before lodging an appeal with the AAT.
Information for Medical Practitioners
Informed consent
The use of ‘unapproved’ therapeutic goods is considered experimental. The Authorised Prescriber must obtain the informed consent of each patient for whom they prescribe the ‘unapproved’ therapeutic good.
The Authorised Prescriber must advise patients:
- that the TGA has not evaluated the ‘unapproved’ therapeutic good’s safety, quality and efficacy
- of the possible benefits and risks of its use
- of the possibility that there may be unknown side effects of any alternative approved goods.
It is best practice to:
- obtain informed consent in writing
- provide a copy of your informed consent form template to the HREC or specialist college
- keep the signed informed consent form on the patient’s file.
Informed consent forms should not be submitted to the TGA.
Prescribing and using the ‘unapproved’ therapeutic goods
In prescribing the ‘unapproved’ therapeutic good for a patient, you are responsible for considering the benefits and risks that apply for the patient. As ‘unapproved’ therapeutic goods have not been evaluated by the TGA, you should remain informed of changes to the benefits and risks as they arise. You should also prescribe the goods in accordance with the legislative requirements relevant to your state or territory.
Obtaining the ‘unapproved’ therapeutic goods
As an Authorised Prescriber, you are responsible for obtaining the ‘unapproved’ therapeutic good. You can do this by contacting the sponsor of the good to arrange supply; however, the sponsor is not legally obligated to supply the good. You can also ask a pharmacy or supplier to arrange supply of the ‘unapproved’ therapeutic good.
You must give the sponsor a copy of your TGA approval letter. This authorises them to legally supply the good for use.
You must also consider whether the good is controlled under the Customs (Prohibited Import) Regulations 1956 and the Customs (Prohibited Export) Regulations 1958, and, if the good is controlled, obtain a permit to import it from the Office of Drug Control.
Access to medicinal cannabis may have additional requirements. For further information, refer to the TGA web page discussing Accessing medicinal cannabis for a patient.
If you are supplying the unapproved therapeutic good in a hospital, you might need any hospital drugs and therapeutics committees to approve the use and funding of these goods within the institution.
Unapproved therapeutic goods are not subsidised under the Pharmaceutical Benefits Scheme (PBS), so you should consider the cost that will be incurred.
Six monthly reporting
It is a condition of the Authorised Prescriber scheme that medical practitioners provide supply reports on the number of patients treated for each of their Authorised Prescriber approvals for the periods 1 January to 30 June and 1 July to 31 December. We must receive these reports within one month of the reporting period ending.
There are two categories to report:
- Number of new patients commenced on treatment or number of devices supplied
- Number of total patients treated during this period.
If no patients have been treated in the relevant period, this must also be reported. The online system will not allow you to submit your report if any of the fields are left blank. If you have not prescribed for any patients using your approval during the reporting period, you must enter zero for new and total patients in order for the report to be submitted.
No new Authorised Prescriber applications will be processed until all outstanding reports are completed and submitted.
Authorised Prescriber six monthly reports should be submitted through the SAS & Authorised Prescriber Online System.
Information on using the online system to submit six monthly reports is found in the Special Access Scheme & Authorised Prescriber Scheme Online system guidance document.
Reporting adverse events and product defects
‘Unapproved’ therapeutic goods generally have not been evaluated for safety, quality and efficacy and could pose unknown risks. Authorised Prescribers are responsible for reporting adverse events or defects arising from the use of ‘unapproved’ therapeutic goods accessed under the Authorised Prescriber scheme.
If you become an Authorised Prescriber, you must report any suspected adverse events or product defects related to the ‘unapproved’ therapeutic good to us within 15 calendar days of learning of it.
You are also required to report any fatal or life threatening adverse drug reactions to the TGA within 7 calendar days after becoming aware of the information and follow up with a complete report if necessary within 8 additional calendar days.
The HREC, specialist college and the good’s sponsor may also require you to provide them with adverse event reports.
There are various ways to report adverse events and product defects, which can be found on our website at Reporting adverse events.
Revoking authorisation
TGA can revoke your Authorised Prescriber status if:
- the HREC or specialist college withdraws their approval or endorsement of your status
- you do not meet the conditions we apply to your approval
- a suitable alternative good becomes available and is entered on the ARTG
- we become aware of any significant concerns about the safety of an unapproved therapeutic good.
If a suitable alternative good is available in the ARTG
If a suitable alternative good becomes available in the ARTG, you should stop using the ‘unapproved’ therapeutic good.
If you want to continue using the ‘unapproved’ therapeutic good you must submit a new Authorised Prescriber application to the TGA.
If applicable, you will need to submit a clinical justification to your evaluating HREC or specialist college to explain why you want to use the ‘unapproved’ therapeutic good instead of the now approved good. The HREC or specialist college will consider this and decide whether continued approval or endorsement is appropriate in light of any available approved good (that has been thoroughly evaluated for safety, quality and efficacy). You must then resubmit this approval or endorsement to the TGA in a new application to become an Authorised Prescriber.
Review of medicines with an established history of use
The TGA will periodically review the ‘unapproved’ therapeutic goods included in subregulations 12B(1B) and 12B(1C) of the Therapeutic Goods Regulations 1990 to determine if additional therapeutic goods or indications need to be added.
We will also remove certain ‘unapproved’ therapeutic goods or indications from the list where necessary, for example, this may be where the therapeutic good become registered in Australia or if a safety concern arises. These may be administrative or clinical decisions based on the ongoing monitoring of the scheme.
Sponsors and health practitioners cannot apply to the TGA to have goods included or removed from the list. At this time, only medicines have been included in the list.
Information for HRECs and specialist colleges
HRECs and specialist colleges provide the initial assessment of a medical practitioner’s application to become an Authorised Prescriber for therapeutic goods not included in subregulation 12B(1B) or 12B(1C) of the Therapeutic Goods Regulations 1990.
Details of what should be reviewed are outlined in Applying for HREC or specialist college approval or endorsement. By undertaking this assessment, you are determining that the use of the ‘unapproved’ therapeutic good is suitable for the proposed indication and that the medical practitioner has the appropriate expertise or qualifications for the proposed use of the ‘unapproved’ therapeutic good. The medical practitioner is ultimately responsible for determining whether it is appropriate to prescribe the ‘unapproved’ goods for each patient that they treat.
HRECs and specialist colleges can approve or endorse a medical practitioner’s application for Authorised Prescriber status. As an HREC or specialist college, you need to determine what level of evidence is appropriate to support an application based on a number of factors, including those described below under Clinical justification for the use of the goods.
Specialist colleges may decide to develop a protocol by which they will evaluate a medical practitioner’s submission. Potential protocols include:
- establishing clinical justification through the use of a clinical practice guideline, which you will require the medical practitioner to follow
- seeking advice from a specialist society which you will consider in your assessment.
The medical practitioner may want to discuss their application with you before they submit it, to determine what level of evidence you will require.
Ensure that only eligible practitioners can access the authorised prescriber scheme. Use the searchable table to confirm whether the prescriber requesting approval or endorsement is eligible to access unapproved therapeutic goods via the AP pathway.
Clinical justification for the use of the goods
The TGA encourages the use of approved therapeutic goods that have been assessed for safety, quality and efficacy (listed on the ARTG). You need to evaluate the clinical justification the medical practitioner provides to determine whether the use of the ‘unapproved’ therapeutic good is appropriate, considering the availability of any approved goods.
The medical practitioner must provide:
- details of the indication for which the good will be used
- a clinical justification for its use.
You should consider whether the clinical justification is appropriate, with regard to:
- the seriousness of the condition
- expected benefits of the proposed treatment versus the potential risks
- approved treatments for the same indication:
- have they been attempted or used?
- why are they inappropriate?
- will they be trialled prior to prescribing the ‘unapproved’ therapeutic good?
- why is the proposed ‘unapproved’ therapeutic good a more appropriate treatment?
- how the risk associated with the use of an ‘unapproved’ therapeutic good will be managed
- the monitoring that will be undertaken
- the process of investigating and reporting adverse events.
The following are not justifications for the use of an ‘unapproved’ therapeutic good:
- that the ‘unapproved’ good is less expensive than any approved treatment
- personal preference for an ‘unapproved’ good.
Providing approval or endorsement
If you support the medical practitioner’s application, provide the medical practitioner with an approval letter if you are a HREC or a letter of endorsement if you are a specialist college. This letter must declare that you have reviewed all the necessary documentation.
You may apply conditions to the approval or endorsement. If the medical practitioner does not meet these conditions, you may revoke your approval or endorsement. Past conditions have included requirements to:
- provide regular reports on how the ‘unapproved’ therapeutic good is used, such as the periodic report the medical practitioner submits to the TGA, which outlines the number of patients that have been treated in a 6 month period
- report suspected adverse events to you within a specified timeframe.
The medical practitioner must provide the TGA with a copy of the letter of approval or endorsement (where required) so we can determine if it is appropriate to approve them as an Authorised Prescriber. They will also supply these documents to the ‘unapproved’ therapeutic good’s sponsor, thereby authorising them to supply the goods.
The letter of endorsement or approval should include the following details, which must match the information provided on the application form:
- a clear statement that approval or endorsement is being given for the purpose of the medical practitioner becoming an authorised prescriber
- the name of the medical practitioner who has gained approval or endorsement
- the sites at which use is covered by the approval or endorsement
- any conditions that have been applied to the approval or endorsement
- a declaration that all necessary documentation has been reviewed.
- The declaration that all necessary documentation has been reviewed may be either included in the letter or approval or endorsement, or as a separate document.
- the unapproved therapeutic goods for which approval or endorsement has been given
- Note, medicinal cannabis products are accepted under the following categories:
- Schedule 4 medicinal cannabis products
- Schedule 8 medicinal cannabis products
- Note, medicinal cannabis products are accepted under the following categories:
- The letter must specify the dosage form, route of administration, indication and class of patients for approval/endorsements.
- The letter must be signed by the chair of the approving ethics committee or an appropriate representative of the endorsing specialist college.
To assist HRECs or specialist colleges with preparing a letter of approval or endorsement, we have provided the below template as a guide. Use of this template is optional and at the discretion of the HREC or specialist college.
An acceptable format for a medicinal cannabis category based HREC approval would be as follows:
| Dosage form: | Oral liquid | Oils | Extracts | Tinctures | Wafers | Lozenges |
|---|---|---|---|---|---|---|
Route of administration:
| Oral | Oral and/or Sublingual | Oral | Oral | Sublingual | Sublingual |
Indication/s: (NB: Only list indications appropriate to that dosage form, route of administering and schedule of product) | For example
|
|
|
|
|
|
| Dosage form: | Oral Liquid | Oils | Extracts | Tinctures | Wafers | Lozenges |
|---|---|---|---|---|---|---|
Route of administration:
| Oral and/or vaporisation and/or inhalation | Oral and/or Sublingual and/or Inhalation | Oral | Oral | Sublingual | Sublingual |
Indication/s:
|
|
|
|
|
|
|
| Dosage form: | Resins | Dried flowers | Dried herbs | Crystals |
|---|---|---|---|---|
Route of administration:
| Inhalation | Inhalation and/or vaporisation | Inhalation and/or vaporisation | Inhalation and/or vaporisation |
Indication/s:
|
|
|
|
|
Applications not approved or endorsed
If you do not approve or endorse an application, provide the medical practitioner with the reason for your decision in writing.
Withdrawal of approval or endorsement
You may withdraw your approval or endorsement of a medical practitioner if it is no longer appropriate.
This could include circumstances where:
- the medical practitioner has not met the conditions that you have applied to their approval or endorsement
- you become aware that the medical practitioner is using the ‘unapproved’ therapeutic good inappropriately
- a suitable alternative good becomes available in the ARTG and authorised for supply in Australia
- you become aware of significant concerns about the safety of the unapproved therapeutic good.
If you withdraw your approval or endorsement of a medical practitioner, inform the TGA of this decision.
Information for sponsors
Releasing ‘unapproved’ therapeutic goods to medical practitioners
A sponsor should be satisfied that the prescriber holds a valid TGA approval letter before the release of an ‘unapproved’ therapeutic good.
Reporting requirements
Sponsor reporting obligations under the Therapeutic Goods Regulations 1990 (Regulation 47B) are as follows.
Sponsors are reminded that they must report to us every 6 months in relation to unapproved therapeutic goods supplied under the SAS and Authorised Prescriber schemes.
Regulation 47B of the Therapeutic Goods Regulations 1990 outlines the requirement for the sponsor (importer) to submit six-monthly supply reports to the TGA listing the product (trade name) details and quantities supplied in Australia in the relevant period. Reporting periods are 1 January - 30 June (inclusive) and 1 July - 31 December (inclusive). Reports must be submitted within one month of the end of the relevant reporting period.
Sponsor six monthly reporting data for medicinal cannabis products are used to publish medicinal cannabis product details by active ingredient category. This list aims to support health care professionals in prescribing and supplying medicinal cannabis products therefore timely submission of six monthly reports is essential.
Please complete the below template and email the report to SAS.Support@health.gov.au.
See the approved Sponsor six monthly reporting form.
Reporting adverse events and product defects
We encourage sponsors to report all adverse events and product defects to us. This helps us to monitor the safety of all therapeutic goods.
However, sponsors are expected to report:
- fatal or life-threatening adverse reactions to us early - ideally within 7 calendar days of becoming aware of them and then follow up with a more complete report within the next 8 calendar days
- other serious and unexpected adverse reactions - within 15 calendar days and advise us if you think any of these may have already been reported to us.
Advise us as soon as possible of any information that could affect the risk-benefit assessment of the product or situations in which the product should be used.
There are various ways to report adverse events and product defects, which can be found on our website at Report a problem or side effect.
Legal basis of the scheme
Therapeutic goods in Australia are regulated under the Therapeutic Goods Act 1989 (the Act), the Therapeutic Goods Regulations 1990 (the Regulations) and the Therapeutic Goods (Medical Devices) Regulations 2002 (the Medical Devices Regulations). Under the Act, only goods entered in the ARTG can be legally supplied in Australia.
Under section 19 of the Act, some medicines are exempt from inclusion in the ARTG. Similarly, some biologicals and medical devices are also exempt under subsection 32CM and chapter 4, parts 4–7 of the Act, respectively. These provisions allow for the Authorised Prescriber scheme.
Medicines
The following clauses relate to the Authorised Prescriber Scheme and access to ‘unapproved’ medicines:
- subsection 19(5) of the Act provides that a specific medical practitioner may be authorised to supply a medicine to a specified class or classes of patient
- regulation 12B of the Regulations relate to medicines and provide that:
- you must be a medical practitioner and have approval from an appropriate ethics committee to become an Authorised Prescriber
- if the medical practitioner does not have access to an appropriate ethics committee, they may seek endorsement from a specialist college with relevant expertise
- if the medical practitioner wishes to apply for a medicine that is included in subregulation 12B(1B) or 12B(1C) of the Regulations, then ethics committee approval or specialist college endorsement is not required to be submitted to the TGA
- the medical practitioner may prescribe an ‘unapproved’ therapeutic good only for patients with a life-threatening or otherwise serious illness or condition
- the medical practitioner must meet any conditions applied to their approval as an Authorised Prescriber.
- subsection 31B(3) of the Act provides that a medical practitioner who has been approved under subsection 19(5) may be notified in writing that they must provide information on matters including the:
- supply of the goods
- handling of the goods
- monitoring of the supply of the goods
- results of the supply of the goods.
Biologicals
The following clauses relate to the Authorised Prescriber Scheme and access to ‘unapproved’ biologicals:
- subsection 32CM(1) of the Act provides that a specific medical practitioner may be authorised to supply a biological to a specified class or classes of patients
- regulation 12C of the Regulations relate to biologicals and provides that:
- you must be a medical practitioner and have approval from an appropriate ethics committee to become an Authorised Prescriber
- if the medical practitioner does not have access to an appropriate ethics committee, they may seek endorsement from a specialist college with relevant expertise
- the medical practitioner may prescribe an ‘unapproved’ therapeutic good only for patients with a life-threatening or serious illness
- the medical practitioner must meet any conditions applied to their approval as an Authorised Prescriber
- subsection 32JG(3) of the Act provides that a medical practitioner who has been approved under subsection 32CM(1) may be notified in writing that they must provide information on matters including the:
- supply of the biological
- handling of the biological
- monitoring and supply of the biological
- results of the supply of the biological.
Medical devices
The following clauses relate to access to ‘unapproved’ medical devices and the Authorised Prescriber Scheme:
- subsection 41HC of the Act states that, subject to the requirements of the Medical Devices Regulations, a specific medical practitioner may be authorised to supply specific kinds of medical devices to a specified class of patient. Conditions may be applied to this authority
- regulation 7.6 of the Medical Device Regulations states:
- you must be a specialist medical practitioner approved by an appropriate ethics committee to become an Authorised Prescriber
- if the specialist medical practitioner does not have access to an appropriate ethics committee, they may seek endorsement from a specialist college with relevant expertise
- the class of patients for which the medical practitioner may prescribe an ‘unapproved’ therapeutic good must have a life-threatening or serious illness or condition
- regulation 1.3 of the Medical Devices Regulations adopts the Health Insurance Act 1973’s definition of a ‘specialist’ to generally mean a medical practitioner who is a fellow of the relevant college in relation to the speciality (other than general practice)
- regulation 7.7 of the Medical Device Regulations states the medical practitioner must meet any conditions applied to your approval as an Authorised Prescriber
- subsection 41JF(1) of the Act provides that a medical practitioner who has been approved under subsection 41HC may be notified in writing that they must provide information on matters including the:
- supply of devices of those kinds
- handling of devices of those kinds
- monitoring of the supply of devices of those kinds
- results of the supply of devices of those kinds.
Prohibition of promoting ‘unapproved’ therapeutic goods
The Act provides (at Section 22(6) for medicines and biologicals and section 41MM for medical devices) that a person must not publicly claim they can supply ‘unapproved’ therapeutic goods.
Information and privacy
The TGA meets our privacy requirements under the Department of Health’s Privacy Policy, the Privacy Act 1988 and the Freedom of Information Act 1982.
Related links
Page history
Link to HREC approval or specialist college endorsement letter template.
- Removed ‘Changes to the Authorised Prescriber Scheme’ section
- Updated to clarify the medical practitioner registration types that can apply to become an Authorised Prescriber
- Email update for sponsor reporting
Title changed from 'Authorised Prescriber Scheme' to 'Becoming an authorised prescriber for unapproved therapeutic goods in Australia' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Minor updates relating to the SAS and AP Online System.
- minor updates under reporting requirements
- link and email updates for sponsor reporting.
- minor updates under reporting requirements in section 22
- link and email updates for sponsor reporting.
Review and addition of information relating to subregulation 12B(1C), six monthly reporting and use of the online portal.
- updated to include guidance on regulatory amendment and application pathways
- other minor updates to clarify list of HRECs, types of medical practitioners, specialist college definition, adverse event terminology, six monthly reporting.
- new title
- new material aligned with MMDR Review recommendations
- restructured.
Access to ‘unapproved’ Therapeutic Goods (Authorised Prescriber Scheme).
Original publication.
Link to HREC approval or specialist college endorsement letter template.
- Removed ‘Changes to the Authorised Prescriber Scheme’ section
- Updated to clarify the medical practitioner registration types that can apply to become an Authorised Prescriber
- Email update for sponsor reporting
Title changed from 'Authorised Prescriber Scheme' to 'Becoming an authorised prescriber for unapproved therapeutic goods in Australia' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Minor updates relating to the SAS and AP Online System.
- minor updates under reporting requirements
- link and email updates for sponsor reporting.
- minor updates under reporting requirements in section 22
- link and email updates for sponsor reporting.
Review and addition of information relating to subregulation 12B(1C), six monthly reporting and use of the online portal.
- updated to include guidance on regulatory amendment and application pathways
- other minor updates to clarify list of HRECs, types of medical practitioners, specialist college definition, adverse event terminology, six monthly reporting.
- new title
- new material aligned with MMDR Review recommendations
- restructured.
Access to ‘unapproved’ Therapeutic Goods (Authorised Prescriber Scheme).
Original publication.

