Download this resource
Manufacturers must demonstrate their medical device complies with the Essential Principles.
Manufacturers must generate, collate, assess, and maintain scientific and clinical evidence that shows their devices comply with the Essential Principles.
Evidence must be relevant to the device's intended purpose and must be objective, sufficient, and robust.
The checklist is a template to help you:
- identify the safety and performance requirements that apply to your device
- document a rationale for any of the safety and performance requirements that aren't relevant
- summarise the evidence you hold in support of each of the relevant safety and performance requirements
Completing the checklist can help you:
- apply for an Australian conformity assessment certificate
- determine if you have considered and addressed all the requirements with supporting evidence
Using the checklist
Your checklist should:
- be completed for each new medical device conformity assessment application
- provide an updated checklist for conformity assessment substantial change applications relating to your medical device
- be applicable to the subject medical devices or may be completed for a family or group of devices
Filling out the checklist
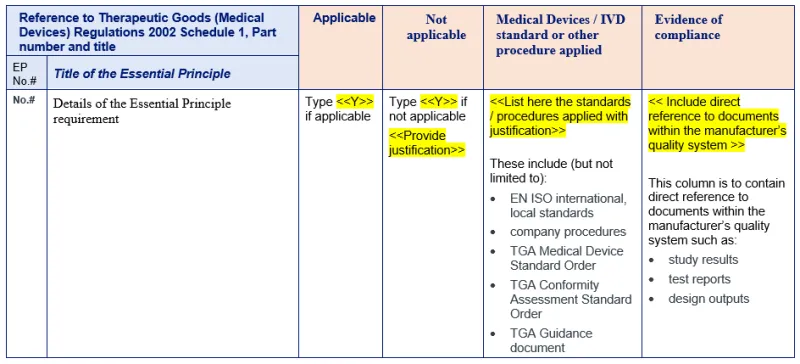
Next steps
- Manufacturers should keep completed Essential Principles checklists on file for use when required.
- Completed checklists can be provided to the TGA as part of conformity assessment applications.
Page history
Updated checklist with the following new sections:
- EP13(13.5) - UDI device identifier and UDI production identifier.
- EP13(13.6) - medical device packaging identifier.
- EP13C - rules for UDI medical devices.
Following section updated for UDI compliance:
- EP13(13A.2) - patient implant cards for implantable devices.
Updated checklist to fix various formatting and accessibility issues:
- Remove unnecessary restrictions and security limitations that did not allow data to be entered correcting into the tables as required.
- URL links have been attached to the main principles; these links will take you directly to the principles associated TGA webpage.
- The checklist now includes a 'products' line where you can add which product the checklist applies too. This is helpful for when you need to fill out numerous checklists.
Removal of PDF documentation due to formatting errors and issues. Essential Principles checklist now available via Microsoft Word attachment.
Updated checklist to include updated Essential Principles.
Original publication.
Updated checklist with the following new sections:
- EP13(13.5) - UDI device identifier and UDI production identifier.
- EP13(13.6) - medical device packaging identifier.
- EP13C - rules for UDI medical devices.
Following section updated for UDI compliance:
- EP13(13A.2) - patient implant cards for implantable devices.
Updated checklist to fix various formatting and accessibility issues:
- Remove unnecessary restrictions and security limitations that did not allow data to be entered correcting into the tables as required.
- URL links have been attached to the main principles; these links will take you directly to the principles associated TGA webpage.
- The checklist now includes a 'products' line where you can add which product the checklist applies too. This is helpful for when you need to fill out numerous checklists.
Removal of PDF documentation due to formatting errors and issues. Essential Principles checklist now available via Microsoft Word attachment.
Updated checklist to include updated Essential Principles.
Original publication.

