Highlights and key insights
The Influenza Batch Release and Testing Program the southern hemisphere 2025 season determined the following findings:
- Total of 62 seasonal influenza vaccine batch release requests were processed by the TGA from seven products available (Afluria Quad, Fluad Quad, Flucelvax Quad, Influvac Tetra, Fluquadri, Fluzone High-Dose Quad and Vaxigrip Tetra; See: 2025 Seasonal influenza vaccines | Therapeutic Goods Administration (TGA)).
- Assessed 17 seasonal batches (27.4% of supplied batches) of influenza vaccines for HA content by SRID (see Table 1).
- All influenza vaccine batches tested by SRID complied with potency specifications.
- Four batches were placed on a monitoring program to ensure HA stability throughout shelf-life.
- Testing at 6 months showed no decrease in HA content indicating acceptable stability of HA.
- Confirmed that all batches supplied on the Australian market met manufacturing requirements based on review of manufacturing data.
- One batch was released using the OCABR pathway, the first time for an influenza product.
- Four batches experienced minor temperature excursions (range 0.7-16.6°C) from the approved shipping conditions of 2-8°C, which were assessed as acceptable based on temperature degradation stability study data.
Introduction and purpose
Seasonal influenza vaccines registered in Australia currently contain 4 strains of virus – two type A strains (H1N1 and H3N2) and two type B strains (B/Victoria and B/Yamagata lineage viruses). The seasonal composition of the vaccine changes on an annual basis to account for the rapid antigenic changes to the influenza virus. Multiple manufacturing platforms are utilised to produce these products.
For the southern hemisphere (SH)2025 season, 6 egg-derived (split virion and surface antigen) and one cell-derived product were available. Compared to the SH2024 influenza vaccine composition, one strain change was made; the H3N2 strain changed from an A/Thailand/8/2022 (H3N2)-like to a A/Croatia/10136RV/2023 (H3N2)-like strain.
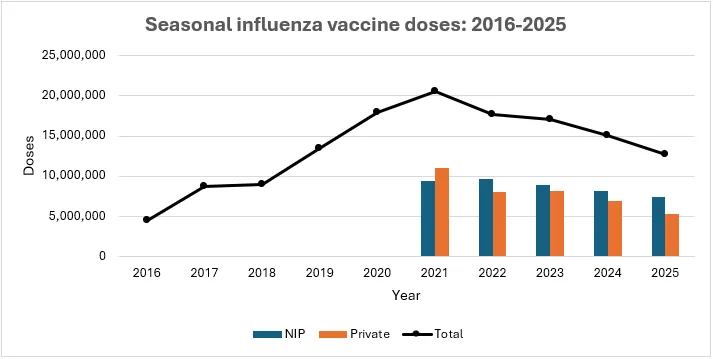
For SH2025, over 12.7 million doses of influenza vaccines were supplied on the Australia market. Of the supplied doses, 5.34 million doses were designated for the private market and 7.38 million doses for the National Immunisation Program (NIP), which provides seasonal influenza vaccines to pregnant women, children under 5 years of age and to adults with specific medical conditions.
A decrease in doses available on the Australian Market for SH2025 compared to SH2024, and notably the peak in SH2021, is consistent with a return to the pre-COVID-19 pandemic levels of influenza vaccine (see Figure 1). The decline is mainly in the private market. The number of doses available is sufficient to meet demand.
Figure 1: Total number of seasonal and pre-pandemic influenza vaccine doses released by TGA from 2016-2025.
The SH2025 Influenza Vaccine Batch Release program aims to verify that influenza vaccines available in Australia contain the correct strains, the appropriate amount of biologically active viral HA content for potency and to identify any quality issues that may indicate a safety signal.
Method
The Single Radial Immunodiffusion (SRID) assay is performed on selected batches to determine HA content of egg- and cell-derived vaccines as per the European Pharmacopoeia guidelines (Ph. Eu. 07/2022:0158, 07/2022:0869 and 07/2022:2149).
The product must meet the confidence limit specifications of a lower confidence limit (P = 0.95) is not less than 80 per cent of the amount stated on the label for each strain to be acceptable.
For products with a label claim of 15 µg HA/strain/dose (Afluria Quad, Fluad Quad, Flucelvax Quad, Influvac Tetra, Fluquadri and Vaxigrip Tetra), that equates to an LCL of 12 µg/strain/dose. For Fluzone High-Dose Quad with a label claim of 60 µg HA/strain/dose, the LCL is 48 µg/strain/dose.
Test results are analysed using the European Directorate for Quality of Medicines and Healthcare (EDQM) Combistats: Application for the Statistical Analysis of Biological Dilution Assay Results or Potency Assay Results (see: CombiStats). The Combistat template includes a completely randomised multiple-dose assay design with slope ratio (dose) model, quantitative response with observed residuals and no transformation. The assay results from the 3 samples of each batch tested are combined to generate potency estimates. Confidence limits are calculated for a 95% confidence level. Then using unweighted geometric combination estimate potency results and Lower Confidence Limit (LCL) are reported.
Testing results are assessed in conjunction with review of manufacturer data for all batches to ensure both consistency of production and compliance with approved testing specifications. An exception to TGA review is when a batch is released using the European Union Official Control Authority Batch Release (OCABR) for Human Biologicals (Pathway 1). Overseas certification or test reports are used as evidence that the batch has already undergone independent testing and assessment by a recognised National Control Laboratory, similar and aligned to the assessment undertaken by the TGA.
Additional data, including shipping temperate conditions and product packaging (cartons and vial labels), are also assessed during the program.
Samples
Seven seasonal vaccine products were assessed for supply in the SH2025 season to the Australian market. Where available, three batches of every product supplied were selected for laboratory testing.
Results
All batches tested by SRID met HA content specifications (see Table 1).
Table 1: Summary of batches tested and outcome during SH2025 Influenza vaccine Batch release program.
Sponsor | Product Name | ARTG | Batch No | Test Outcome Pass/ Fail |
|---|---|---|---|---|
Seqirus | Afluria Quad | AUST R 294907 | 398153 | Pass |
Seqirus | Afluria Quad | AUST R 294907 | 398169 | Pass |
Seqirus | Afluria Quad | AUST R 294907 | 399981 | Pass |
Seqirus | Fluad Quad | AUST R 313724 | 398189 | Pass |
Seqirus | Fluad Quad | AUST R 313724 | 398199 | Pass |
Seqirus | Fluad Quad | AUST R 313724 | 398205 | Pass |
Seqirus | Flucelvax Quad | AUST R 319093 | 398207 | Pass |
Seqirus | Flucelvax Quad | AUST R 319093 | 398208 | Pass |
Seqirus | Flucelvax Quad | AUST R 341450 | 3047926 | Pass |
Sanofi | FluQuadri | AUST R 213963 | U8646DA | Pass |
Sanofi | FluQuadri | AUST R 213963 | U8658BA | Pass |
Sanofi | FluQuadri | AUST R 213963 | U8709CA | Pass |
Sanofi | Vaxigrip Tetra | AUST R 299922 | 5DA51D1 | Pass |
Sanofi | Vaxigrip Tetra | AUST R 299922 | 5DA34D1 | Pass |
Sanofi | Vaxigrip Tetra | AUST R 299922 | 5DA94D2 | Pass |
Sanofi | Fluzone HD Quad | AUST R 320962 | U8648BA | Pass |
Viatris | Influvac Tetra | AUST R 292237 | K06 | Pass |
Conclusion
Given the annual changes in influenza vaccine strains, it remains essential to ensure that consistent, high-quality seasonal Influenza vaccines are available on the Australian market. The influenza batch release testing program confirms correct formulation, assesses production consistency, flags products requiring further evaluation due to low HA results and monitor batch stability.
The SH2025 Influenza Batch Release Testing Program confirmed that all batches of influenza vaccine tested met the required potency specifications. Consistency in manufacturing and production processes was also confirmed across all products, and no quality concerns were identified for any influenza vaccine products supplied for the SH2025 season.
For the 2026 season, egg-derived trivalent vaccines (TIV) products will re-enter the Australian market in accordance with WHO guidance to remove the B/Yamagata strain from the formulation. Products holding an existing TIV ARTG number will not require modifications to the current testing program; however, new products will undergo comprehensive batch testing to establish product characteristics and quality.
Based on test results and a thorough review of seasonal influenza vaccine manufacturing data, the influenza vaccines available in Australia are confirmed to be safe and produced to the high standards expected of biological products. They are suitable for all eligible Australians and are recommended to prevent serious infection, thereby protecting both individuals and the broader community.

