Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance helps sponsors with the process of applying for orphan drug designation of a prescription medicine. They can receive a waiver of application and evaluation fee for registration on the Australian Register of Therapeutic Goods (ARTG).
For information on the eligibility criteria and supporting documentation requirements for orphan drug designation, please see the guidance on eligibility criteria.
Legislation
Process for orphan drug designation
Flowchart showing the process for orphan drug designation
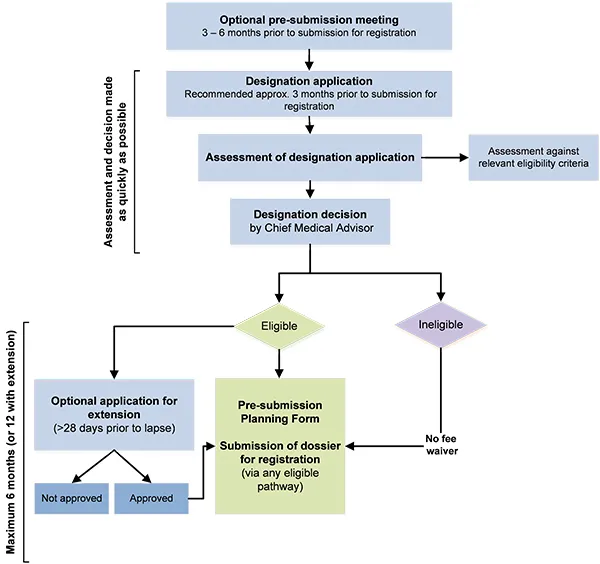
The image shows a flowchart depicting the process for submitting an application for registration, with various steps and decision points. The flowchart is divided into two main sections: the upper part focuses on the assessment and decision process, while the lower part deals with the outcomes and further steps.
The process begins with:
- Optional pre-submission meeting (3-6 months prior to submission for registration)
- Designation application (Recommended approx. 3 months prior to submission for registration)
- Assessment of designation application, which includes a side step for "Assessment against relevant eligibility criteria"
- Designation decision by Chief Medical Advisor
The flowchart then branches based on the decision:
If "Eligible":
- Leads to "Pre-submission Planning Form"
- Then "Submission of dossier for registration (via any eligible pathway)"
If "Ineligible":
- Leads directly to "Submission of dossier for registration (via any eligible pathway)" with a note of "No fee waiver"
An additional branch from "Eligible" shows:
- "Optional application for extension (>28 days prior to lapse)"
- This further branches into "Not approved" and "Approved"
- Both of these outcomes lead back to "Submission of dossier for registration"
The left side of the flowchart indicates that the upper section (assessment and decision) should be completed "as quickly as possible," while the lower section (from eligibility decision to submission) has a "Maximum 6 months (or 12 with extension)" timeframe.
This flowchart illustrates the steps and timelines involved in the application and assessment process for registration, including optional steps and potential outcomes.
What is orphan drug designation
Purpose of designation
Designation is a formal process that allows us to make a decision under regulation 16J of the Therapeutic Goods Regulations 1990 (the Regulations) regarding whether the medicine is eligible for orphan drug designation.
The designation application precedes the registration application and is the formal application made using a specified form requesting assessment against the relevant eligibility criteria and a decision from TGA.
Application and evaluation fees are waived for prescription medicine registration applications if a related orphan designation is in force.
You can lodge an application for registration of an orphan drug through any available prescription medicine registration process, subject to meeting the requirements for that registration process.
An orphan drug designation does not guarantee registration on the Australian Register of Therapeutic Goods (ARTG).
Benefits of designation
A valid orphan drug designation must be held in order for benefits resulting from designation to apply to a submission for registration on the ARTG.
Orphan drug designation of a medicine can:
- facilitate orphan drug access to the Australian marketplace
- help offset orphan drug development costs
- provide a consistent and transparent process for assessment against the eligibility criteria
Medicines with orphan drug designation have the same evidence requirements as other prescription medicines and will be evaluated by TGA for quality, safety and efficacy in the same way.
Eligibility requirements
Eligibility criteria for the orphan drug program are set out in regulation 16J of the Regulations. See our guidance on orphan drug eligibility criteria for more information.
End of transition period
A transition period was in place for orphan drug designations which were made prior to the reform of the program, at a time when their status would not lapse. In accordance with regulation 54 of the Regulations, these designations remained in force for a period of 12 months from 1 July 2017 - i.e. until 30 June 2018, to allow sufficient time for stakeholders to adjust to the new program.
Specific transition arrangements were also provided in regulation 55 of the Regulations for those applications for orphan drug designation that were pending at the time the amendments which introduced the new program commenced.
Orphan designations under the previous program will remain published on the TGA website but are no longer in force with the end of the transition period.
Designation application and assessment
See the Process for orphan drug designation flowchart for an overview of the process.
Arranging a pre-submission meeting - step 1
You may request a pre-submission meeting with TGA to discuss a planned orphan drug designation application and subsequent submission for registration. These meetings should occur six months prior to the date you plan to submit your registration application (and therefore should occur up to three months prior to lodging your designation application). The content and procedures for these meetings are as per the standard prescription medicines pre-submission meetings process.
Details of any pre-submission discussions should be provided in the Pre-submission Planning Form (PPF) and the dossier (Module 1.7.1), as per the usual process.
Access to TGA Business Services (TBS) - step 2
If you already have a client ID number and password to access TGA Business Services (TBS) as a Submitter, go to the next step.
Obtaining access to TBS
Designation applications are created and lodged by people with Submitter access through the TGA Business Services portal.
You will need the following in order to access the portal, create and submit applications:
- a TGA Client Identification (Client ID) number
- password access to our TGA Business services portal
- submitter access to the TBS portal
If you do not have a client ID number or access to our business services:
- go to our TGA Business Services: getting started with TGA and submit the online organisation details form.
Submitting your designation application- step 3
We recommend that you submit your application for designation three months prior to the date you plan to lodge your submission for registration.
Under section 16H(2) of the Regulations, to be eligible, your designation application must:
- be submitted using the approved form (the designation/determination application e-form in TBS)
Using the designation application e-form
You can access the designation/determination application e-form through your TBS account- external site. See the designation/determination application e-form guidance for further guidance on filling out the form.
Please see the eligibility criteria and supporting documentation guidance for information on meeting the eligibility criteria and the requirements for supporting documentation to be attached to your designation application.
For any attachments, use the format outlined in Part A and Part B of the General dossier requirements for prescription medicines.
Ensuring that active ingredient(s) have an approved name
When recording the active ingredient(s):
- Check each active ingredient has one of the following approved terms:
- Australian Approved Name (AAN)
- Australian Approved Biological Name (ABN)
- Use the approved ingredient name(s) in the application for orphan drug designation
If the active ingredient(s) is not on the approved list, you can submit a proposal for a new ingredient name to the TGA before, or in parallel to, your application for orphan drug designation.
- Make an application for an approved name using the relevant form:
- Use the proposed name (AAN/ABN) in the application form for orphan drug designation, including the word 'proposed' in brackets after the name.
Note: Applications for orphan drug designation cannot be finalised until an approved name has been allocated to the active ingredient(s).
Fees for orphan drug designation
There is no fee for an orphan drug designation application.
You will need to pay the determination application fee if you are submitting an application for orphan drug designation at the same time as an application for priority determination or provisional determination. This fee will be refunded if your orphan drug designation is approved. See the priority determination or provisional determination step-by-step guidance for more information.
TGA assessment of the designation application - step 4
We will assess your designation application and make a decision as quickly as possible.
After you have submitted your designation application using the e-form, we will check the information provided and confirm whether it is sufficient to allow us to assess the application.
We will check that your application:
- form has been completed in full, including declarations
- meets the administrative requirements
- has separately addressed each eligibility criterion with clear, robust and scientific justifications
- contains justifications that are up to date
- includes supporting data and references where required.
We will not formally notify you of the results of this checking process, however, you may receive requests for further information.
If you have any questions during the designation application assessment period, please contact AET.Application.Entry.Team@health.gov.au.
How we will assess your designation application
We will review your application for designation against the eligibility criteria set out in sub-regulation 16J (3) or (4) of the Regulations, depending on whether you have applied for a medicine that is not a new dosage form medicine or a medicine that is.
The focus of our assessment is to determine whether the application and supporting documentation establishes that the eligibility criteria are met. We may seek independent expert advice on aspects of the assessment if required.
A recommendation from the relevant Clinical Evaluation Unit will be referred to TGA's Chief Medical Advisor, who is the delegated decision-maker for orphan designation applications.
Requests to the sponsor for additional information
We may request additional information or clarification from you during the course of our assessment. These requests will include a timeframe for response which is determined on a case-by-case basis, and details of TGA contacts for your response. The usual time frame for your response will be approximately five working days.
You are responsible for providing evidence in support of your application.
Any time taken for you to respond to requests will extend the overall assessment and decision-making timeframe. If no response is received in the specified time period, TGA will make a decision based on the available information.
Withdrawing your designation application
You may withdraw your designation application at any time before we notify you of our decision.
To withdraw the application, email AET.Application.Entry.Team@health.gov.au.
Your email should include:
- a statement that you wish to withdraw an orphan drug designation application
- the designation application number
- the active ingredient(s)
- the sponsor's name
- the proposed indication for designation.
We will not publish on our website details of applications for determination that are withdrawn before the decision is made or are assessed as ineligible for determination.
Notifying sponsors of the designation decision - step 5
If we assess that your application for designation meets the eligibility criteria, the medicine will be designated as an orphan drug.
After we have made a decision, we will advise you via email of the outcome as soon as practicable (see sub-regulations 16J (6) and 16J (7)).
Your decision letter will include:
- the name of the sponsor of the medicine
- a statement of reasons for designation decisions (for ineligible decisions only)
- the name of the medicine
- each active ingredient of the medicine to which the designation relates
- the orphan indication
- the dosage form
If the delegate decides to refuse to designate the medicine as an orphan drug, in addition to the items above, your decision letter will also include:
- reasons for the decision to refuse to designate the medicine as an orphan drug
- details of your appeal rights.
How to appeal the designation decision
The orphan drug designation decision is appealable under Regulation 48.
Appeals regarding designation decisions must be lodged within 90 days of the designation decision being issued.
You can seek internal review by TGA or external review by the Administrative Appeals Tribunal.
Publication of designation outcomes
If an orphan drug designation is approved, we will publish details of the eligible designation decision on our website at Prescription medicine determinations and designations (subregulation 16J (5)).
Publication will include:
- the name of the medicine
- the sponsor's name
- the eligible orphan indication
- the dosage form
- designation decision date and lapsing date
Designation decisions will be published as soon as you have been notified.
We will not publish details of designation applications that are assessed as ineligible or those that are withdrawn before we make a decision.
Period during which the designation is in force
Your orphan drug designation comes into force when it is made and remains in force for six months (refer sub-regulation 16K (1) of the Regulations) unless it is extended or revoked. The period during which the designation is in force is not affected by lodgement of a related registration application.
Note: Applications to the Pharmaceutical Benefits Advisory Committee (PBAC) link to the TGA orphan drug designations for certain fee waivers to apply. Sponsors intending to make a concurrent or subsequent application to the PBAC should consider the implications of any orphan drug designation ceasing to be in force, prior to any such application.
Inquiries regarding applications to PBAC should be directed to PBAC@health.gov.au
Applying for extension of designation
If you are unable to lodge your submission for registration within the initial 6 month period of validity, you may apply to TGA in writing for one extension of your orphan drug designation only. Approved extensions will be granted for a further six months, as set out in sub-regulation 16L (1).
Applications for extension of orphan drug designation must be lodged in the approved form using the designation/ determination extension application eForm on TGA Business Service- external site at least 28 calendar days before the designation lapse date. Applications made less than 28 days before the designation lapse date will not be accepted for assessment (see sub-regulation 16L (2)(b)).
See our guidance on completing a designation or determination extension application form in TGA Business Services for more information on filling out the form.
In the application form and supporting documents, you must:
- explain why you are seeking an extension and provide justification of why the extension will allow you to lodge a registration application within the extension period, AND
- provide updated justification that:
- there are no existing therapeutic goods for diagnosis, prevention or treatment of the condition in question, OR
- if such therapeutic goods exist, that the medicine will bring improved efficacy, safety or a major contribution to patient care to those affected, AND
- provide a current update on the approval status of the medicine by the regulatory agencies specified in regulation 16J(3) or (4) as applicable. The medicine may no longer be eligible if an application has been refused by one of these regulatory agencies due to the medicine’s safety AND
- provide an update on any other aspects of your designation application that have changed since the original designation application
An orphan drug designation cannot be extended if TGA has previously extended the designation (sub-regulation 16L (4)(a)).
Fee for orphan drug designation extension applications
There is no fee for applications to extend orphan drug designation.
Assessment of the extension application
Similar to the original designation application, we may request additional information or clarification from you throughout our assessment of your designation extension application.
As outlined in sub-regulation 16L (4), we will make a decision regarding whether your orphan drug designation should be extended based on whether the delegate is satisfied that:
- you have not previously applied for an extension of the designation
- the relevant eligibility criteria are still met (also see application for extension)
- refusal to approve on the grounds of safety
- comparison against registered therapeutic goods; and
- granting an extension would be likely to result in you lodging a submission for registration within the extension period
- assessment is against the eligibility criteria set out in sub-regulation 16J (3)(e and f) or 16J (4) (d and e) of the Regulations, depending on whether you have applied for a medicine that is not a new dosage form medicine or a medicine that is.
Notification of outcomes - Application for extension
We will notify you of the outcome of your application for extension via email as soon as possible after the decision has been made by TGA.
Approved extension letters will include details of the extended orphan drug designation period.
If your application for extension is not approved, you will be provided with a statement of reasons for the decision.
Decisions regarding a refusal to extend an orphan drug designation can be appealed under Regulation 48.
Only one extension will be granted per orphan drug designation.
Revocation of orphan drug designation
TGA reserves the right to revoke the designation prior to the end of the designation period under Regulation 16M.
This may occur if:
- you request that the TGA revoke the designation. The process for applying for revocation of a valid orphan drug designation before lapsing is the same as withdrawing your designation application, OR
- the Secretary is satisfied that the medicine no longer meets the relevant eligibility criteria (either new dosage form under sub-regulation 16J (3), or non-new dosage form under sub-regulation 16J (4)).
However, we will not routinely review designation decisions during their validity.
If we decide to revoke your designation, we will advise you of the decision and the reasons for the decision in writing as soon as practicable.
Decisions regarding revocation of designations can be appealed under regulation 48 of the Regulations.
Re-lodgement of lapsed designation
If you wish to obtain the orphan drug fee waiver and your designation including any extensions have lapsed, you must submit a new designation application.
We will re-assess the new designation application against the eligibility criteria, as some aspects such as ‘comparison against existing therapeutic goods' may have changed since the original designation decision was made.
You are required to provide the reference number/s for previous designation applications in your new application for designation.
Further assistance
If you have read the guidance and still require assistance, contact: AET.Application.Entry.Team@health.gov.au.
Submitting your application for registration - step 6
An application for registration of a designated orphan drug can be lodged through any of the available prescription medicines registration pathways. Guidance on submitting your PPF is available on the TGA website.
Waiver of registration fees
To be eligible for a waiver of registration fees a related orphan designation for the medicine, indication, dosage form and sponsor must be in force when the relevant fee is payable. The application fee is payable at the time of making an application for registration and the evaluation fee is payable on the day on which the applicant is notified of the amount of the evaluation fee (following preliminary assessment).
Fees are payable if the orphan designation for the medicine has lapsed, or the application is outside of the scope of the orphan designation (e.g. indication or dosage form). There is no requirement for an orphan drug designation to be renewed. You may apply for a new orphan designation at any time if you wish to do so. Consider our guidance eligibility criteria and whether you still address all criteria prior to lodging an application.
Note: Fees for minor variations to the registration of a medicine (i.e. regulatory activities under section 9D of the Act) cannot be waived for an orphan drug.
Combining orphan and non-orphan applications
Submissions for registration that combine applications for which an orphan designation is in force with applications for which no orphan designation is in force will not be eligible for a fee waiver.
Page history
Title changed from 'Orphan drug designation' to 'Applying for orphan drug designation for a prescription medicine' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Minor updates and edits by Prescription medicines authorisation branch.
Original publication.
Title changed from 'Orphan drug designation' to 'Applying for orphan drug designation for a prescription medicine' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Minor updates and edits by Prescription medicines authorisation branch.
Original publication.

