Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
We recognise that sponsors and other information providers may wish to provide information and increase public awareness about health conditions and their management.
These disease education activities can help raise awareness about diseases, aid recognition of symptoms and encourage consumers to seek appropriate advice when necessary.
It is important to distinguish between disease information and advertising.
Advertising of therapeutic goods must be compliant with our requirements. Criminal and civil penalties may apply if you do not meet these legal requirements.
Legislation
Disease education activities
Disease education activities can comprise a broad range of education methods delivered across varied settings:
- campaigns
- seminars and webinars
- workshops, courses and other face-to-face education
- audio-visual education material
- online education resources, including downloadable materials
- community-level resources such as flyers, posters and booklets.
Disease education activities often involve diseases or conditions that require diagnosis, treatment or management by a health professional.
While a disease education activity may make reference to a range of treatment options, if the information provided is likely to encourage consumers to seek to obtain a particular good, or seek a prescription for a particular medicine, then it will be considered an advertisement.
Special care is required for disease-education activities where there are limited treatment options because the information may draw attention to one specific therapeutic good, whether that good is named or not.
Disease education or advertising
The following diagram can assist you to categorise information as either disease education or advertising and identify if legislative requirements apply.
Diagram to help categorise information as disease education or advertising
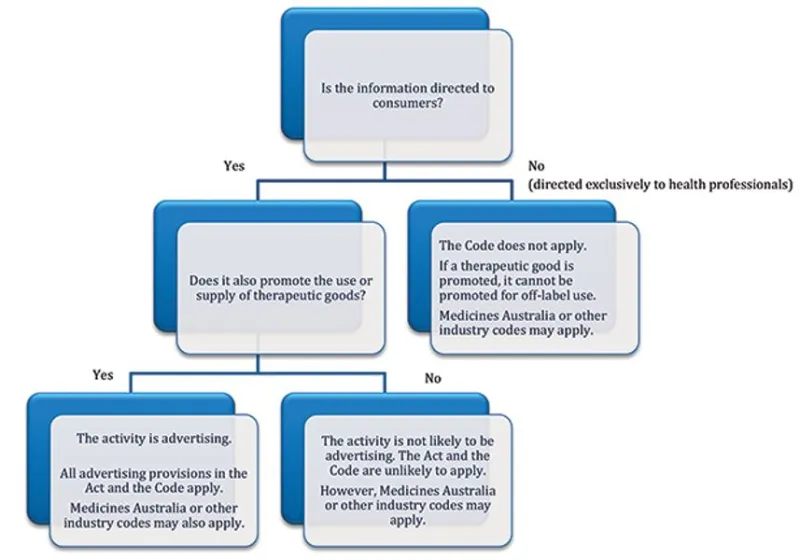
The flowchart starts with the question 'Is the information directed to consumers?' If yes, it proceeds to the next question, 'Does it also promote the use or supply of therapeutic goods?' If yes, the activity is advertising, and all advertising provisions in the Act and the Code apply. If no, the activity is not likely to be advertising, and the Act and the Code are unlikely to apply.
If the information is not directed to consumers, it is directed exclusively to health professionals. The code does not apply. If a therapeutic good is promoted, it cannot be promoted for off-label use. Medicines Australia or other industry codes may apply.
Activities that may constitute advertising
Not all information released to the public about therapeutic goods is advertising.
However, if information directly or indirectly promotes the use or supply of a therapeutic good, from a reasonable consumer’s point of view, then it is likely advertising.
Our guidance on understanding activities that represent advertising is designed to assist you to understand what material may be considered advertising. If content is 'advertising', then the legislative requirements will apply.
Disease education materials do not include promotional information
When presenting disease awareness materials, do not include any other promotional information that may guide consumers to seek out a particular therapeutic good or choose a particular medicine over another.
Examples of promotional information include using:
- testimonials or endorsements
- comparative statements
- promotional claims, such as relating to product efficacy.
When disease education information is provided with advertising material, in context, it is likely to all be considered advertising.
More detailed information can be found in our Guidance on understanding activities that represent advertising.
The nature of the information provider
The nature and purpose of an organisation undertaking non-advertising activities such as disease education contributes to whether a reasonable consumer would consider the information as advertising therapeutic goods.
Disease education activities of organisations with a commercial interest in the manufacture, sale or supply of therapeutic goods are more likely to be seen as advertising.
It is harder to decouple promotional intent from information when presented by a commercially motivated organisation.
Patient support groups
Patient support groups are a type of disease education activity, and the same considerations apply.
As patient support groups contain members of the public, pay particular attention to:
- Our requirements on What can and cannot be advertised to the general public. For example, you cannot advertise prescription medicines to the public.
- Public access to information on prescription-only or some pharmacist-only medicines, including social media. These are forms of advertising and are easily accessible by the public.
See:
Medicines Australia Code of Conduct
When prescription-only medicines are registered in the Australian Register of Therapeutic Goods (ARTG), it is a condition of their registration that any promotional activities comply with the Medicines Australia (MA) Code of Conduct. Non-compliance with the conditions of registration may have serious penalties under the Act.
Page history
Changes made across Guidance to improve readability.
Changes to reflect the requirement for advertising pre-approval in specified media ending on 30 June 2020.
Original publication.
Changes made across Guidance to improve readability.
Changes to reflect the requirement for advertising pre-approval in specified media ending on 30 June 2020.
Original publication.

