Overview
The TGA introduced categories of medicinal cannabis products specifically for the SAS and AP pathways.
Since 22 November 2021, SAS and AP submissions for unapproved medicinal cannabis products have been made under a category based on cannabinoid content, rather than by brand (trade) name. The categories provide greater flexibility to prescribers by allowing the substitution of products and ensure continuity of treatment (for example in the event of product shortage or discontinuation). Each product falls under one of five categories based on cannabinoid content.
See the list of Medicinal cannabis products by SAS and AP category.
Grouping products by cannabinoid content means prescribers do not have to submit applications for individual brands of a product. This is similar to other medicines that can be applied for based on ‘generic’ (active) ingredients.
A list of category names and the product criteria can be found at SAS and AP categories for medicinal cannabis products.
Sponsors
Calculating the category of medicinal cannabis product
The product sponsor is responsible for declaring the correct category for use by health professionals.
Products are grouped based on the proportion of cannabidiol (CBD) content compared with the total cannabinoid content to align with the Poisons Standard. Therefore, all category 1 products should also be Schedule 4 products as defined in the Poisons Standard. Grouping is not based on the psychoactivity or THC concentration of a product.
The total cannabinoid content of a medicine should be determined using a Certificate of Analysis (CoA). All cannabinoids listed in the CoA should be considered as part of the total cannabinoid content, regardless of whether they are defined as an active ingredient in the Therapeutic Goods (Standard for Medicinal Cannabis) (TGO 93) Order 2017.
Examples for sponsors are provided below:
Example 1: Cannabidiol dominant dried herb (flower)
- Certificate of analysis:
- CBD: 12% w/w
- THC: 0.77% w/w
- Other cannabinoids: 1.8% w/w
Calculation:
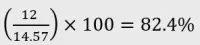
- Category: Category 2 product as CBD is ≥60% and <98% of total cannabinoid content.
Example 2: Cannabigerol isolate oral liquid
- Certificate of analysis:
- CBD: <0.00% w/v
- CBG: 95.50% w/v
- THC: <0.00% w/v
- Concentration: 3mg/mL CBG
Calculation:
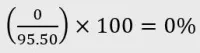
- Category: Category 5 product as CBD is <2% of total cannabinoid content.
Example 3: 1:1 Balanced dried herb (flower)
- Certificate of analysis:
- CBD: 11.7% w/w
- THC: 12.5% w/w
Calculation:
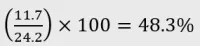
- Category: Category 3 product as CBD is <60% and ≥ 40% of total cannabinoid content.
Example 4: 1:1:1 Oral liquid
- Certificate of analysis:
- CBD: 2.26% w/v
- THC: 2.18% w/v
- CBG: 2.28% w/v
Calculation:
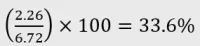
- Category: Category 4 product as CBD is ≥2% and <40% of total cannabinoid content.
NB: CBD = cannabidiol, THC = tetrahydrocannabinol, and CBG = cannabigerol
Health professionals
Prescribers
SAS and AP submissions for medicinal cannabis products are made by category. However, prescriptions must be written for a product. It is the prescriber's responsibility to issue a prescription to the patient in accordance with relevant state or territory legislation.
Prescriptions for medicinal cannabis products:
- must include active ingredient name/s, strength, dosing amount and frequency
- include quantity of medication and number of repeats (if applicable)
- may include brand (trade name) of the medicine where clinically necessary
- may include any other formulation details clinically necessary
- must be in accordance with relevant state and territory legislation
- must be in accordance with the TGA approval
The sponsor of a product must determine the correct category of the product based on cannabinoid content. Prescribers may wish to refer to the list of medicinal cannabis products by SAS and AP category to select a suitable medicine for their patient.
Pharmacists
SAS and AP submissions for medicinal cannabis products are made by category. However, this does not mean pharmacists can substitute between any product included in that category.
In dispensing a medicine, pharmacists:
- must comply with all legislation relevant to the practice of pharmacy in the jurisdiction where the practice occurs
- ensure that dispensing is done in accordance with the prescriber's intentions
- not dispense a prescription without satisfying themselves that it is safe, appropriate and lawful to supply the medicine. Where clarification is required, the patient or their agent should be consulted and if necessary, the prescriber contacted
- undertake brand substitution only in accordance with regular practice and in accordance with the Pharmacy Board of Australia Guidelines for dispensing of medicines
- meet professional standards that are determined by the Pharmacy Board of Australia.
Pharmacists may wish to contact the relevant state and territory health department for further information.
The sponsor of a product must determine the correct category of the product based on cannabinoid content. Pharmacists may wish to refer to the list of medicinal cannabis products by SAS and AP category to determine if the product on the prescription matches the SAS or AP category on the prescriber’s approval letter.

