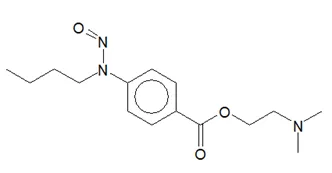
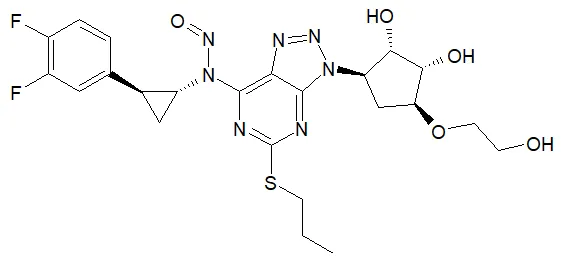
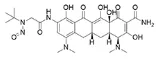
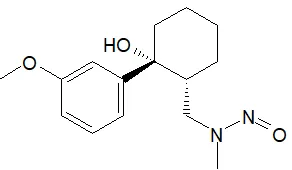
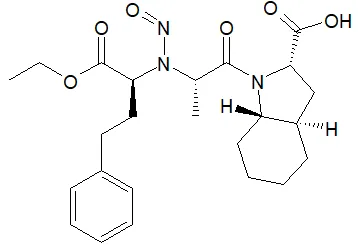
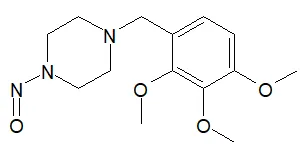
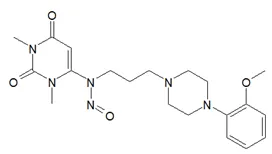
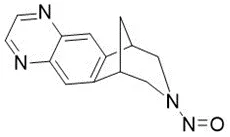
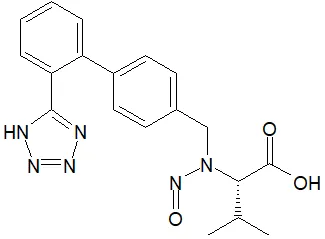
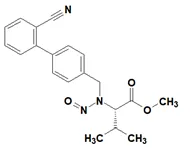
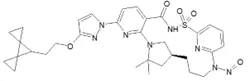
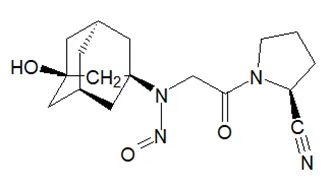
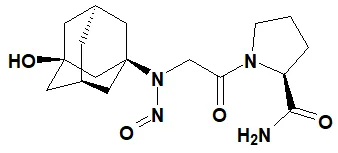
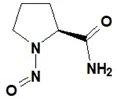
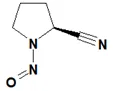
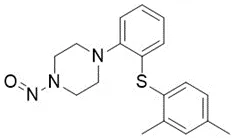
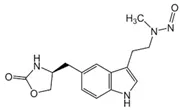
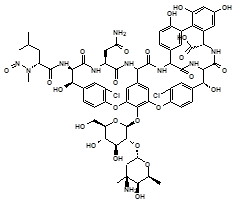
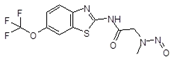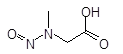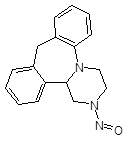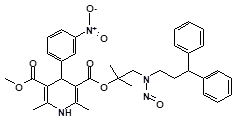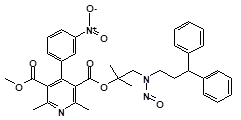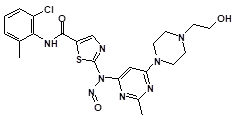Below is a list of established AI limits for some nitrosamine impurities. These apply for all routes of administration.
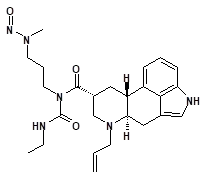
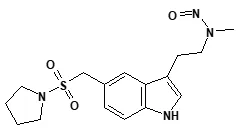
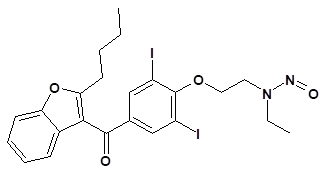
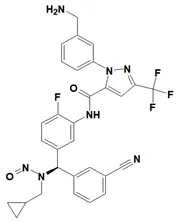
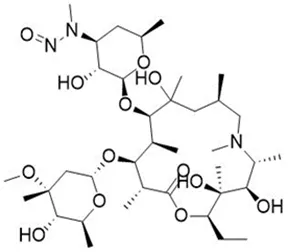
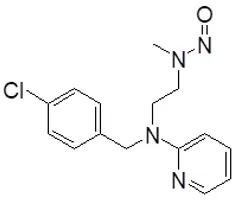
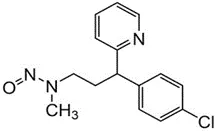
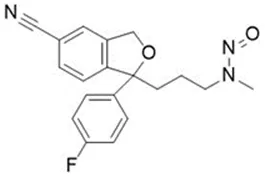
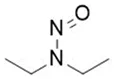
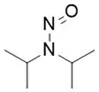
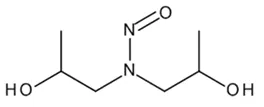
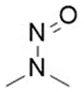
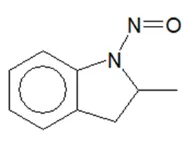
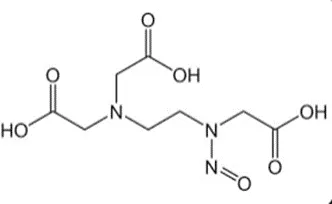
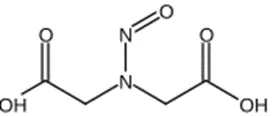
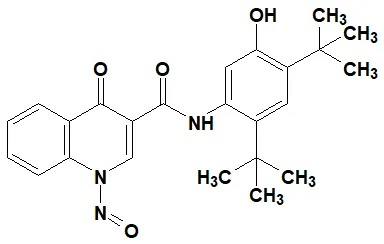
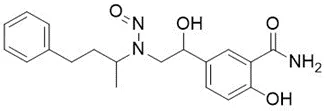
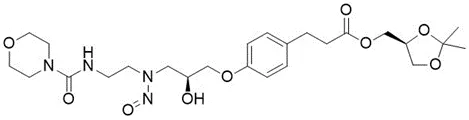
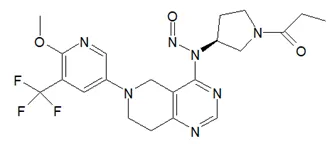
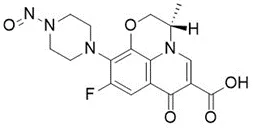
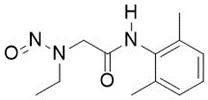
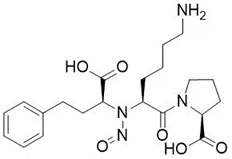
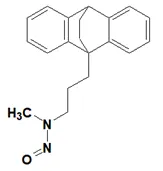
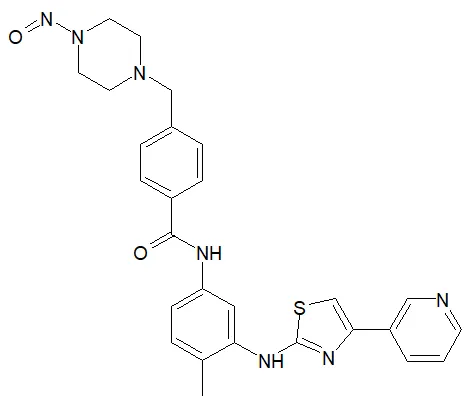
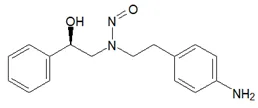
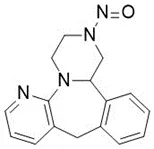
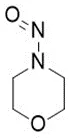
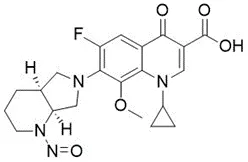
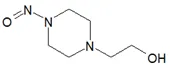
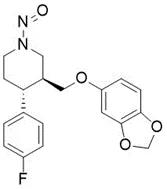
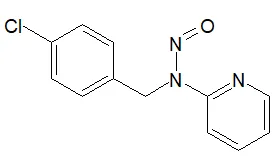
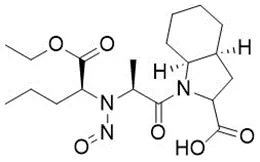
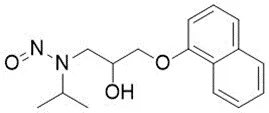
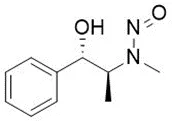
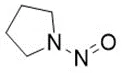
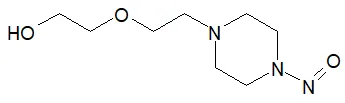
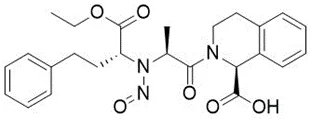
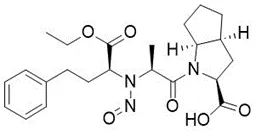
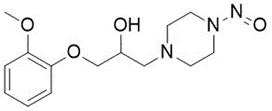
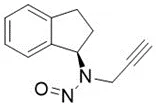
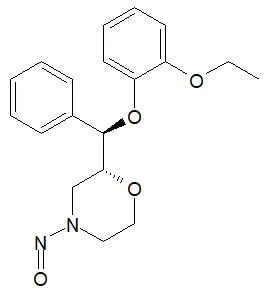
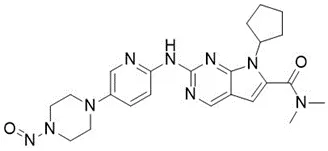
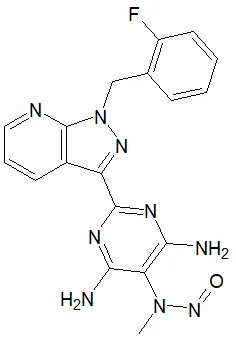
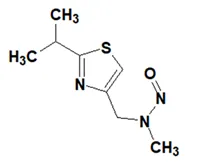
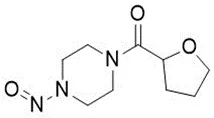
Nitrosamine structures are available in the print version below.
Established AI limits for some other N-nitroso-structures. N-Nitroso-structures that include N-nitrosoguanidines, N-nitroso-indoles and N-nitrosoureas impurities are listed separately.
A revision table listing updates to the AI limit information for nitrosamines and other N-nitroso-structures is available separately.
Established acceptable intake for nitrosamines in medicines
CPCA: Carcinogenic Potency Categorisation Approach
NMI: Non mutagenic impurity should be controlled to ICH Q3A and ICH Q3B guideline limits
- Acceptable Intake (AI) Limit to be applied to maximum daily dose (MDD) of the drug product. These limits are applicable only if a product contains a single N-nitrosamine.
- Potential presence of nitrosamine impurities with structures derived from drug substances or their related impurities. This does not mean that the impurity will be found in all products or pharmaceutical forms containing the drug substance. Please refer to EMA's Q&A on the risk factors for the presence of nitrosamines for details.
Nitrosamine structures are available print version
Established acceptable intake for nitrosamines in medicines
[PDF, 1.09 MB]
Established acceptable intake for nitrosamines in medicines
[DOCX, 1.27 MB]
Product types


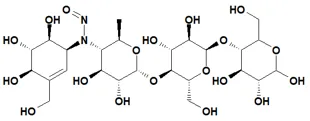
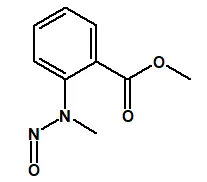
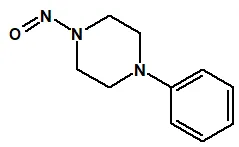
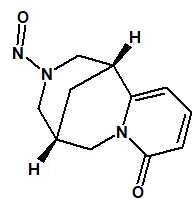
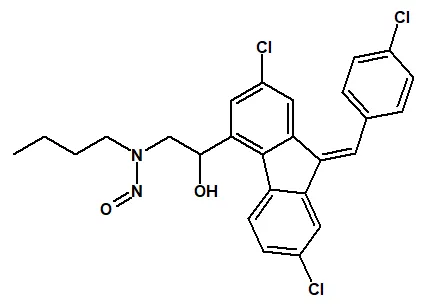
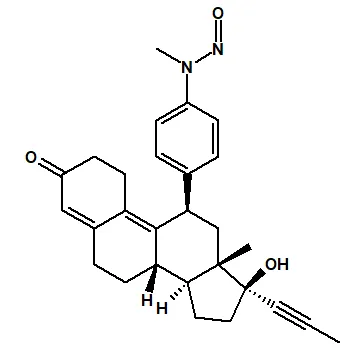
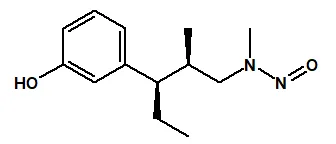
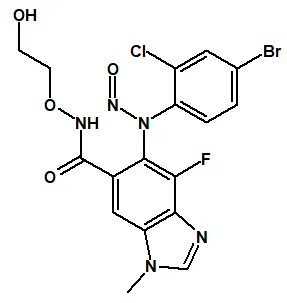
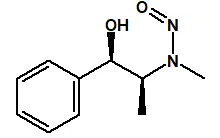
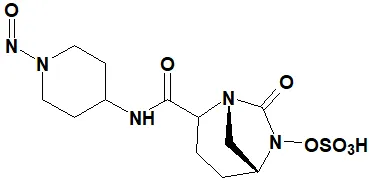
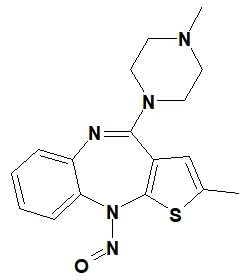
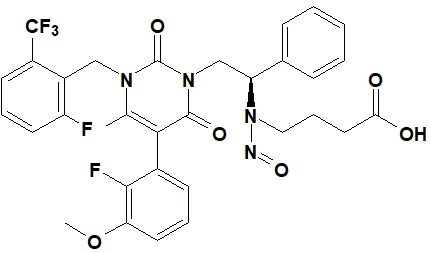
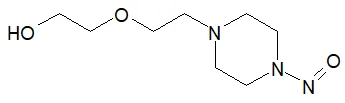
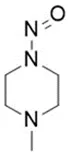
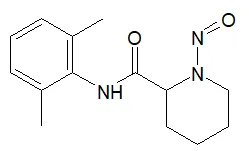
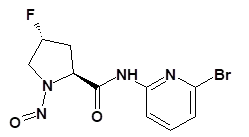
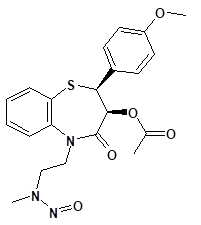
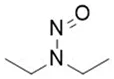
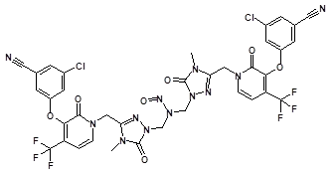
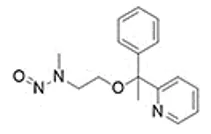
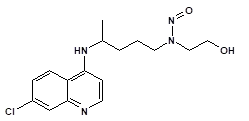
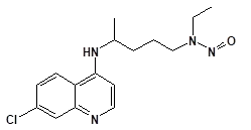
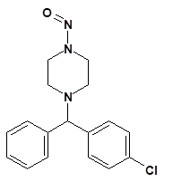
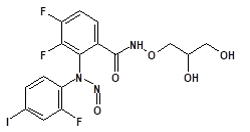
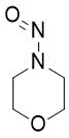
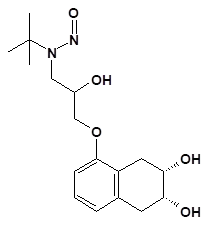
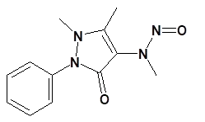
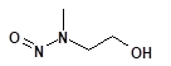
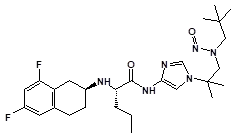
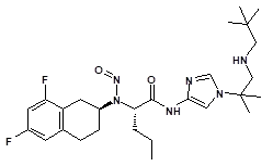
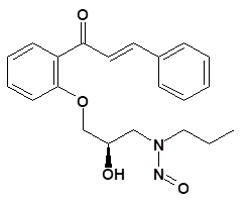
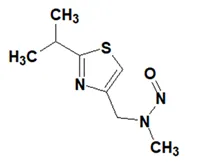
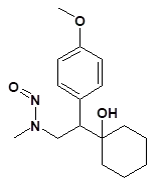
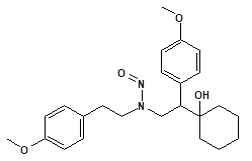
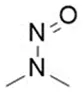
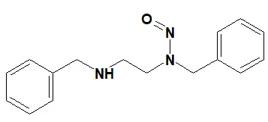
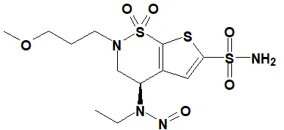
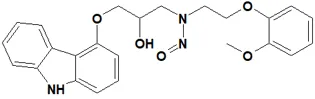
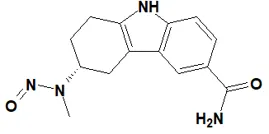
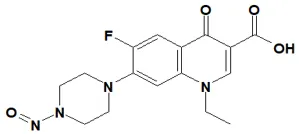
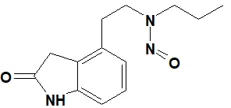
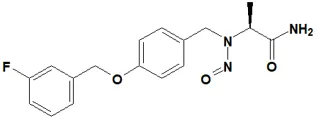
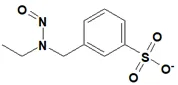
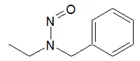
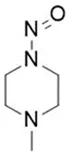
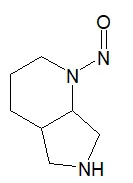
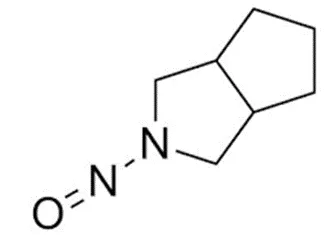
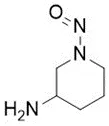
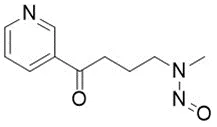
![7-Nitroso-3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo-[4,3-a]pyrazine](/sites/default/files/styles/full_lg/public/2025-01/154%207-Nitroso-3-trifluoromethyl-5%2C6%2C7%2C8-tetrahydro%201%202%204%20triazolo-4%203-a%20pyrazine.PNG.webp?itok=ph8loSIP)
![Nitroso impurity C” [N-(2,6-dimethylphenyl)-2-(4-nitrosopiperazin-1-yl)acetamide]](/sites/default/files/styles/full_lg/public/2025-01/452%20Nitroso%20impurity%20C%20N-2%206-dimethylphenyl-2-4-nitrosopiperazin-1-yl%20acetamide.PNG.webp?itok=eBZuteJc)
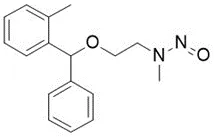
![N-Nitroso-praziquanamine / [2-nitroso-2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one]](/sites/default/files/styles/full_lg/public/2025-01/068%20N-Nitroso-praziquanamine.PNG.webp?itok=2UgWXszZ)
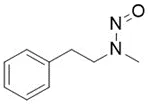
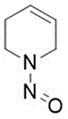
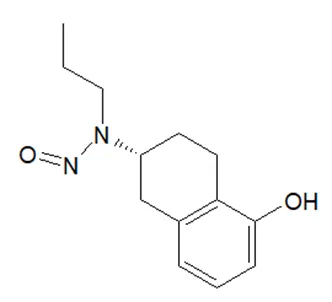
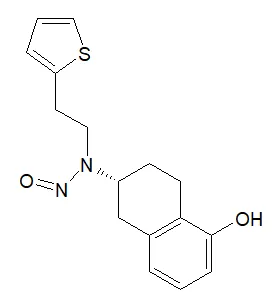
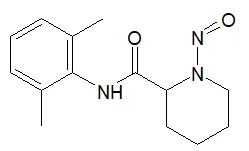
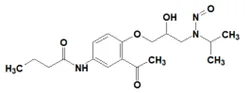
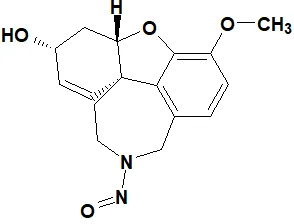
![N-Nitroso-2-(7-methoxy-1-naphthyl)-N-[2-(7-methoxy-1-naphthyl)ethyl]ethylamine](/sites/default/files/styles/full_lg/public/2025-01/120%20N-Nitroso-2-7-methoxy-1-naphthyl-N-2-7-methoxy-1-naphthyl%20ethyl%20ethylamine.PNG.webp?itok=YwYI2fXU)
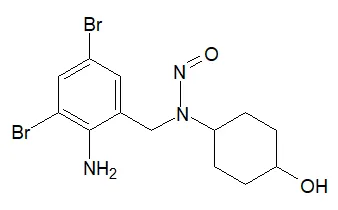
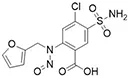
![N-Nitroso-apixaban impurity B / 5-[4-[3-carbamoyl-1-(4-methoxyphenyl)-7-oxo-4,5-dihydropyrazolo[3,4-c]pyridin-6-yl]-N-nitrosoanilino]pentanoic acid](/sites/default/files/styles/full_lg/public/2025-01/143%20N-Nitroso-apixaban%20impurity%20B.PNG.webp?itok=dNlSwbG_)
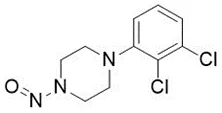
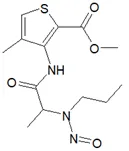
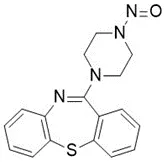
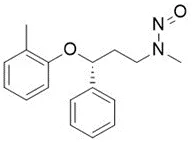
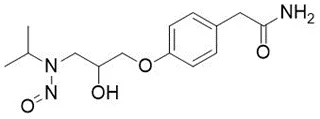
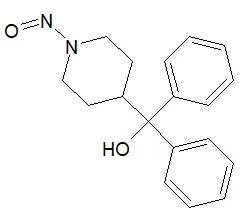
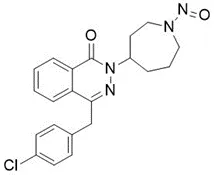
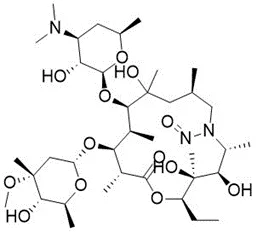
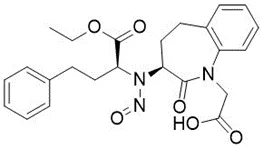
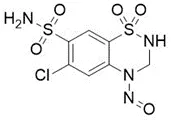
![3-(Dimethylamino)propyl 2-[benzyl(nitroso)amino]benzoate](/sites/default/files/styles/full_lg/public/2025-01/926%203-Dimethylamino%20propyl%202-benzyl%20nitroso%20amino%20benzoate.PNG.webp?itok=ZA3E0FX-)
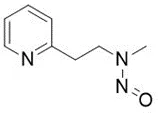
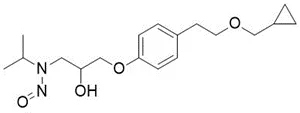
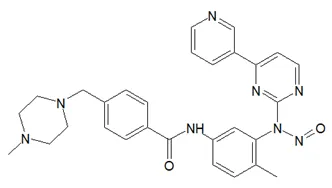
![N-Nitroso-bilastine impurity 2 / 1-(2-ethoxyethyl)-2-(1-nitrosopiperidin-4-yl)-1H-benzo[d]imidazole](/sites/default/files/styles/full_lg/public/2025-01/293%20N-Nitroso-bilastine%20impurity%202.PNG.webp?itok=SBYeS7b_)
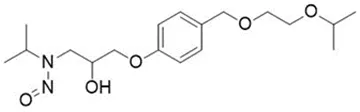
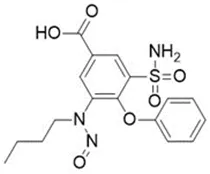
![N-Nitroso-bupropion / N-tert-butyl-N-[1-(3-chlorophenyl)-1-oxopropan-2-yl]nitrous amide](/sites/default/files/styles/full_lg/public/2025-01/373%20N-Nitroso-bupropion.PNG.webp?itok=yfItzc5Q)
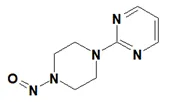
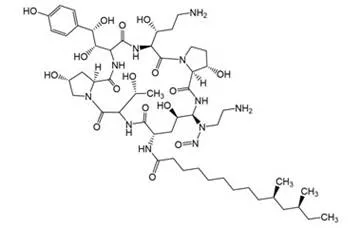
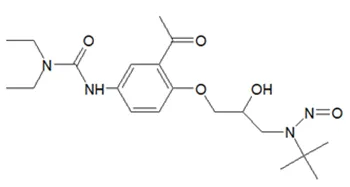
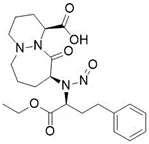
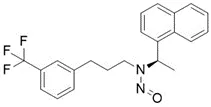
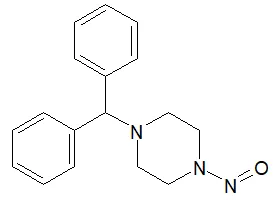
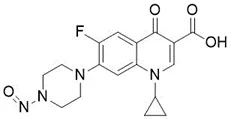
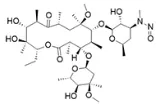
![N-Nitroso-clozapine / 8-chloro-11-(4-methylpiperazin-1-yl)-5-nitroso-5H-dibenzo[b,e][1,4]diazepine](/sites/default/files/styles/full_lg/public/2025-01/726%20N-Nitroso-clozapine.PNG.webp?itok=q8MeFOFP)
![N-Nitroso-clozapine EP Impurity C / 8-chloro-11-(4-nitrosopiperazin-1-yl)-5H-dibenzo[b, e][1, 4]diazepine](/sites/default/files/styles/full_lg/public/2025-01/756%20N-Nitroso-clozapine%20EP%20Impurity%20C.PNG.webp?itok=4QqgfQ3O)
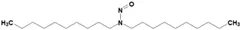
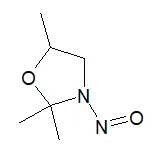
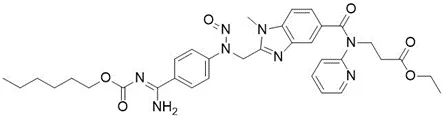
![5-Chloro-4-methyl-2-[(2S)-2-methyl-1-nitrosopyrrolidin-2-yl]-1H-benzimidazole](/sites/default/files/styles/full_lg/public/2025-01/064%205-Chloro-4-methyl-2-2S-2-methyl-1-nitrosopyrrolidin-2-yl-1H-benzimidazole.PNG.webp?itok=fmTUFLJV)
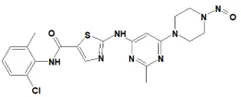
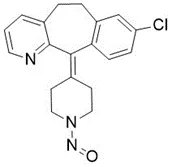
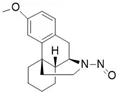
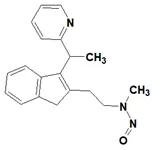
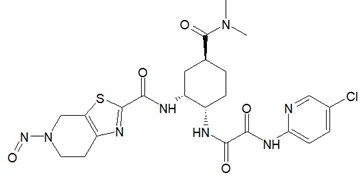
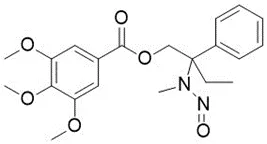
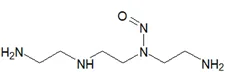
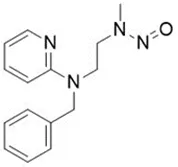
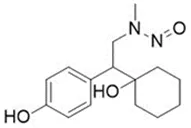
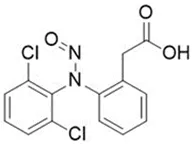
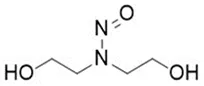
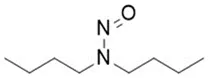
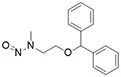
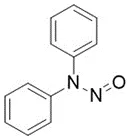
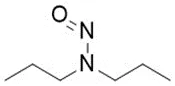
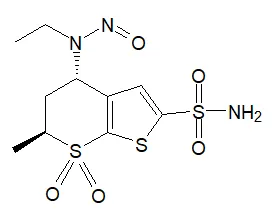
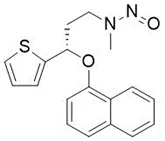
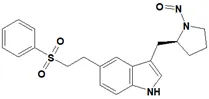
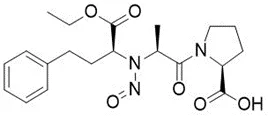
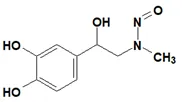
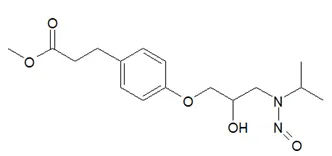
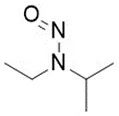
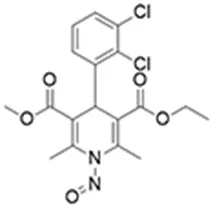
![N-Nitroso-fenfluramine / N-ethyl-N-[1-[3-(trifluoromethyl)phenyl]propan-2-yl]nitrous amide](/sites/default/files/styles/full_lg/public/2025-01/867%20N-Nitroso-fenfluramine.PNG.webp?itok=V3O3EPVC)
![N-Nitroso-ketamine / N-[1-(2-chlorophenyl)-2-oxocyclohexyl]-N-methylnitrous amide](/sites/default/files/styles/full_lg/public/2025-01/078%20N-Nitroso-ketamine.PNG.webp?itok=IpMYkOrq)
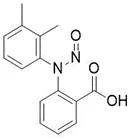
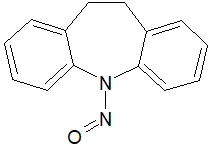
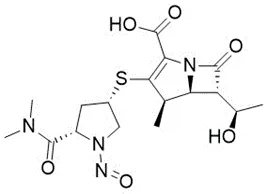
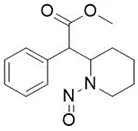
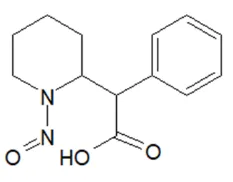
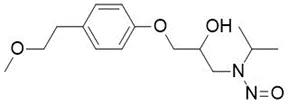
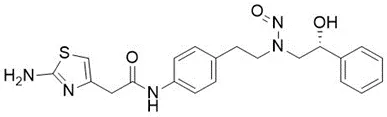
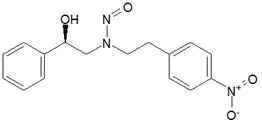
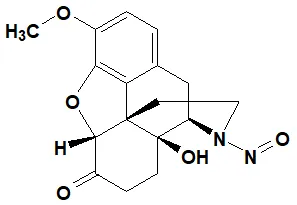
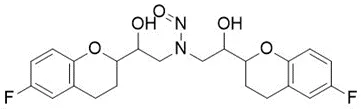
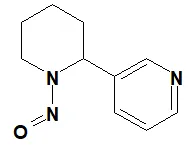
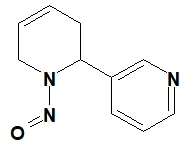
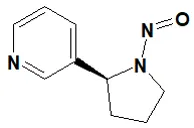
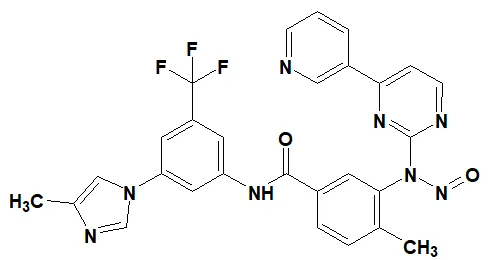
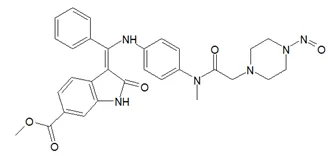
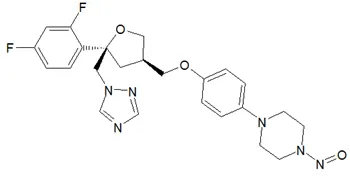
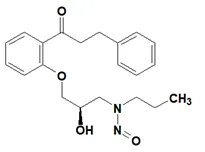
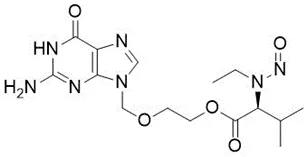
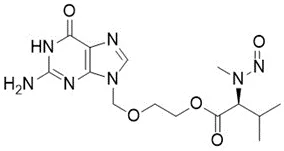
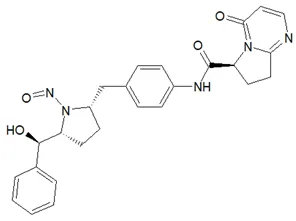
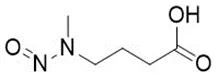
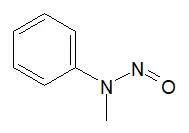
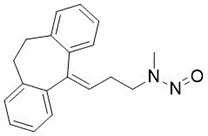
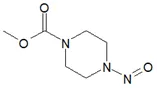
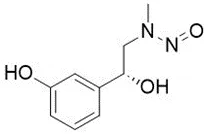
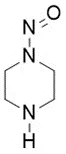
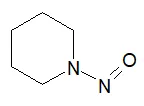
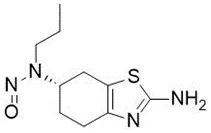
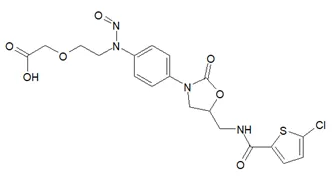
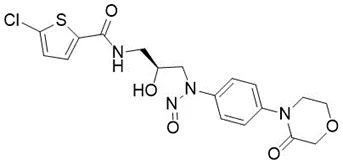
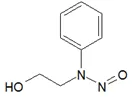
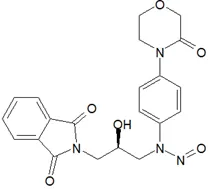
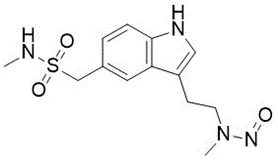
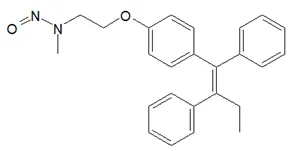
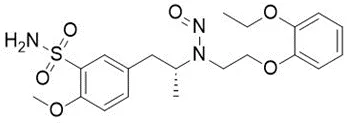
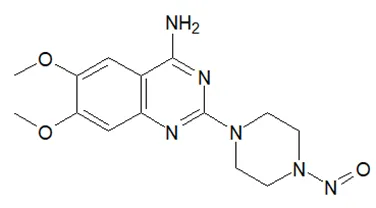
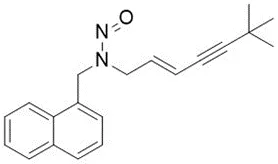
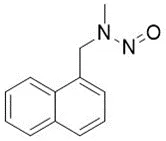
![N-Nitroso-terbinafine degradant N-[(2E)-6,6-dimethyl-2-hepten-4-yn-1-yl]-N-nitrosomethanamine](/sites/default/files/styles/full_lg/public/2025-02/272%20N-Nitroso-terbinafine%20degradant.PNG.webp?itok=vnq5coZn)