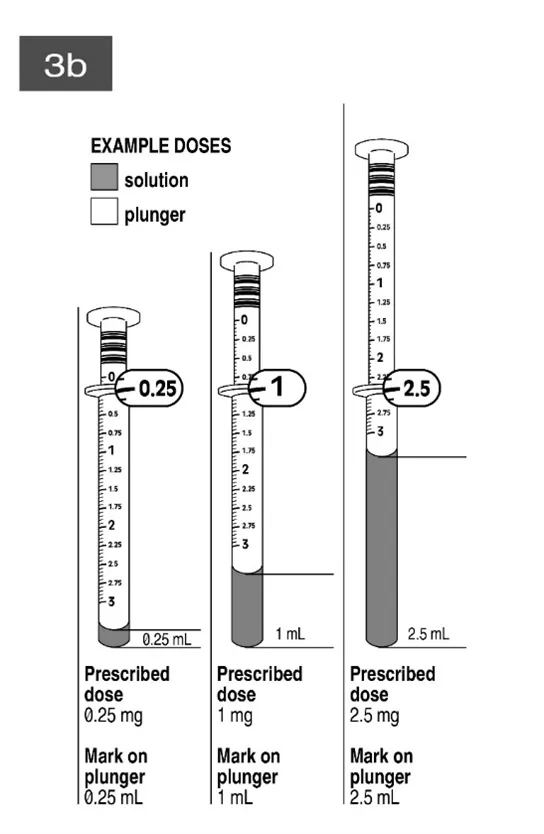
Avoiding paediatric dosing errors with risperidone
Updated illustrations clarify how to use measuring syringe for oral solutions
Published
Related content
-
Clonidine - importance of dosing compliance and safe storage
Medicines Safety Update article -
Administer vinca alkaloids by intravenous infusion only
Medicines safety update - important information for health professionals -
Risk of overdose in infants when using prilocaine/lidocaine cream (EMLA and generics)
We have received 2 serious adverse event cases in neonates in which EMLA (topical prilocaine/lidocaine cream) was applied for a circumcision procedure. Both cases were likely to have involved overdose.