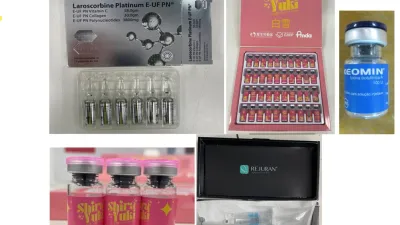
NSW-based individual issued infringement notices for allegedly importing cosmetic injectables
Published
Related content
-
Victorian issued further infringement notices for allegedly importing unapproved therapeutic goods
We have issued 3 infringement notices totalling $11,268 to a Victorian-based individual for allegedly importing unapproved prescription-only medicines. -
Queensland-based individual issued infringement notices for allegedly importing and advertising counterfeit botulinum toxin type A
We have issued 2 infringement notices totalling $7,716 to a Queensland-based individual for the alleged unlawful importation of counterfeit botulinum toxin type A and the unlawful advertising of cosmetic injectables. -
TGA issues infringement notices to 6 individuals for alleged importation and unlawful advertising of therapeutic goods
We have issued 11 infringement notices totalling $43,560, to 6 individuals, for the alleged importation and unlawful advertising of therapeutic goods.