The Australian Government is strengthening patient safety by introducing Unique Device Identification (UDI) for medical devices.
UDI supports the identification of medical devices and other medical device reforms.
It is designed to improve the effectiveness of the regulatory framework, including the management of post-market safety-related activities, such as recalls.
When fully implemented in healthcare, UDI can provide faster identification of devices implanted into patients in the event of an adverse event, safety alert or recall.
UDI can support identification and removal of those devices from storage and distribution to prevent further use.
It also allows patients, consumers, and health professionals to access product information in the Australian UDI Database (AusUDID) about the devices that they use.
The AusUDID provides an easy to access and consistent location for up-to-date product information.
UDI could improve device performance assessment by regulatory bodies, clinical quality registries and device manufacturers through accurate product identification that better supports comparative studies.
With the introduction of UDI, Australia joins a globally harmonised approach that can supports more accurate identification of medical devices.
How UDI works
UDI facilitates traceability through the introduction of a unique device identifier for each model of medical device.
The unique device identifier is a combination of numbers, letters and symbols that show:
- the UDI-Device Identifier (UDI-DI) which indicates the model of medical device
- the UDI-Production Identifier (UDI-PI) which provides the production specific information such as lot or batch number.
The unique device identifier is issued by TGA recognised organisations known as Issuing Agencies.
The Issuing Agencies are responsible for ensuring the UDIs issued are unique and in accordance with global standards.
Device labels and all applicable levels of packaging for applicable devices are required to have a unique device identifier.
The identifiers must be labelled in a way that can be found and read by both people and machines (like a barcode).
The UDI may also be marked directly onto the medical device when the device is intended to be reused on multiple patients, like a scalpel.
Sponsors or manufacturers submit the UDIs and related device data to the AusUDID.
Patients, consumers and healthcare can find the information about the medical device in the AusUDID, by searching using the UDI.
Benefits
Every year, millions of devices are used on or implanted in patients in public, private and day hospitals and clinics.
In some instances, the ability to accurately identify medical implants has been challenging when an issue with an implant has been identified.
UDI allows clear and unambiguous identification of medical devices and facilitates access to information about the device.
This allows better identification of medical devices within Australia, strengthening patient safety and supporting quicker responses to device safety issues.
Further benefits made possible through the adoption of UDI are shown below:
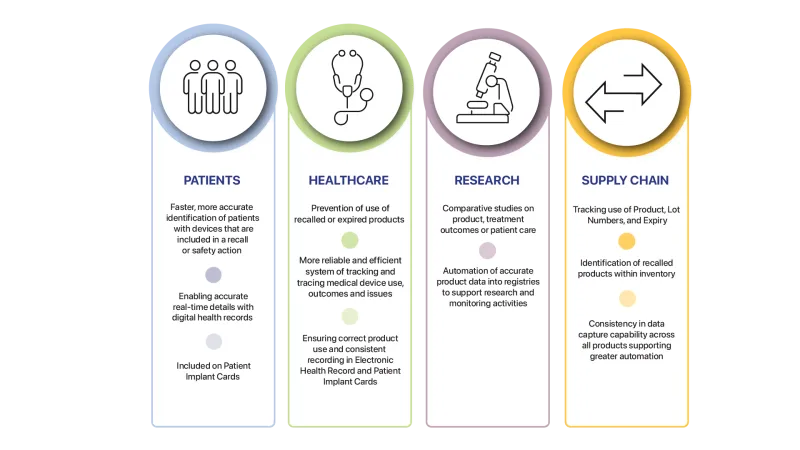
The benefits of UDI for patients, healthcare, research and supply chain are explained below.
Patients:
- Faster, more accurate identification of patients with devices that are included in a recall or safety action.
- Enabling accurate real-time details with digital health records.
- Included on Patient Implant Cards.
Healthcare:
- Prevention of use of recalled or expired products.
- More reliable and efficient system of tracking and tracing medical device use, outcomes and issues.
- Ensuring correct product use and consistent recording in Electronic Health Record and Patient Implant Cards.
Research:
- Comparative studies on product, treatment outcomes or patient care.
- Automation of accurate product data into registries to support research and monitoring activities.
Supply chain:
- Tracking use of product, lot numbers and expiry.
- Identification of recalled products within inventory.
- Consistency in data capture capability across all products supporting greater automation.
The Australian UDI Database (AusUDID)
We have established the AusUDID to store UDI-DIs and related information for devices supplied in Australia.
The data in the AusUDID links to the relevant inclusion(s) in the Australian Register of Therapeutic Goods (ARTG).
Sponsors and manufacturers submit and maintain the device data in the AusUDID. Patients, consumers, clinical quality registries and health professionals can access this information for free.
No patient information is collected or stored in the AusUDID. Patient data is managed by hospitals and healthcare providers.
Learn more about the AusUDID.
Australian UDI implementation timing
We are introducing UDI in phases over a 5-year period with compliance for high-risk medical devices first, followed by lower risk devices over later years.
This allows medical device sponsors or manufacturers to progressively prepare for and comply with UDI requirements.
UDI requirements become mandatory on different dates, based on the risk class of the device or IVD.
High-risk and implantable devices have earlier UDI compliance start dates, with medium and lower risk devices requiring UDI compliance in later years.
The period between when the UDI regulations came into effect and the UDI compliance start date for your device allows you to:
- prepare your organisation
- prepare your device labels and packaging
- prepare your UDI-DIs and related data
- choose and test your methods for submitting data to the Australian UDI Database (AusUDID).
You may voluntarily choose to have your device meet the UDI requirements during this period.
For example, while you must comply with UDI requirements for Class III and Class IIb medical devices from 1 July 2026, you can add UDI labels your devices and submit UDI data to the AusUDID before this date.
If you choose to voluntarily comply with the UDI requirements, we recommend you meet all requirements.
This will reduce confusion for end users such as healthcare.
Learn more about UDI implementation timing.
Enquiries
We have a dedicated UDI Support Team handling enquiries about the Australian UDI implementation and the AusUDID.
The UDI Support Team provides a range of services including:
- Email support during Australian (AET) business hours
- Developing and distributing information and support documents.
The UDI Support Team does not replace the enquiry lines and support channels already offered by us.
Email UDI@health.gov.au
Mailing list
To sign up to our UDI mailing list, contact the UDI Support Team at UDI@health.gov.au.

