Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
We have prepared this guidance to help you as a sponsor or manufacturer understand your regulatory obligations under Australian therapeutic goods legislation. Specifically, this guidance document covers the timing of UDI implementation and when compliance with the UDI requirements will apply.
This guidance complements our guidance on the UDI regulations, which covers:
- medical devices and in vitro diagnostic (IVD) medical devices included in UDI regulations
- labelling requirements
- data submission requirements
- specific device requirements.
For this Guidance:
- we refers to the Therapeutic Goods Administration (TGA)
- you refers to sponsor or manufacturer of medical devices or IVD devices
- UDI record refers to a UDI-Device Identifier (UDI-DI) and related data published as a record to the Australian Unique Device Identification Database (AusUDID)
- UDI compliance start date refers to the date in which you must comply with UDI requirements for your device(s)
- existing devices refers to a device that is manufactured and labelled prior to the UDI compliance start date
- EU certificate refers to an overseas regulator conformity assessment document issued under either of the following (as in force from time to time):
- Council Directive 93/42/EEC of the Council of the European Communities
- Directive 98/79/EC of the European Parliament and the Council of the European Union.
Legislation
UDI outline
The Australian Government is strengthening patient safety by introducing the Australian UDI system for medical devices.
The UDI system supports the identification of medical devices and other medical device reforms. It is designed to improve the effectiveness of the regulatory framework, including management of post-market safety-related activities such as safety alerts and recalls.
By introducing a UDI system, Australia joins a globally harmonised approach that enables more accurate identification of medical devices.
Legislation
The UDI regulatory framework came into effect on 24 March 2025. It includes:
- requirements for medical devices and IVDs to bear a UDI Carrier
- requirements for the submission of UDI records to the AusUDID
- requirements for direct marking of some devices
- dates for each medical device and IVD risk class to meet the UDI requirements.
The requirements for Australia’s UDI were implemented via amendments to:
- the Therapeutic Goods Act 1989 (the Act), and
- the Therapeutic Goods (Medical Devices) Regulations 2002 (the Regulations).
Legislative instruments are amended from time to time and may occasionally be replaced or new instruments made.
UDI compliance timeframes
Introduction
We are introducing UDI over a 5-year period - with compliance for high-risk medical devices first, followed by lower risk devices over later years.
This allows medical device sponsors or manufacturers to progressively prepare for and comply with the UDI requirements.
Figure 1: UDI Compliance timeframes

UDI regulatory framework introduced: 24 March to 30 June 2026
Voluntary labelling of devices and submission of data to Australian UDI Database (AusUDID).
Regulations in effect and AusUDID live: 24 March 2025.
UDI compliance start dates - phased introduction: 1 July 2026 to 30 June 2030
- Phased introduction based on device class, starting with high-risk devices followed by lower risk devices over later years.
- Caters for devices transitioning from the EU's MDD and IVDD to MDR and IVDR.
- Class III and IIb devices supplied during this period and still in the Sponsor's control at the end of the period are to be relabelled.
Phased introduction starts: 1 July 2026.
Full UDI labelling including existing devices (Class III and IIb): 1 January 2030.
Full UDI compliance: 1 July 2030 onwards
- Full UDI compliance.
- Exemptions via consent to supply.
Full Direct Marking: 1 July 2030.
UDI requirements become mandatory on different dates, based on the risk class of the device or IVD. High-risk and implantable devices have earlier UDI compliance start dates, with medium and lower risk devices requiring UDI compliance in later years.1
To help you prepare, we are providing transition arrangements for devices that are:
- manufactured and labelled prior to applicable compliance dates (known as existing devices)
- supplied in Australia under an EU certificate.
For detailed compliance milestones for both medical devices and in vitro diagnostic (IVD) devices, see appendix A below.
Voluntary compliance
The period between when the UDI regulations came into effect and the UDI compliance start date for your device allows you to:
- prepare your organisation
- prepare your device labels and packaging
- prepare your UDI-DIs and related data
- choose and test your methods for submitting data to the Australian UDI Database (AusUDID).
You may voluntarily choose to have your device meet the UDI requirements during this period. For example, while you must comply with UDI requirements for Class III and Class IIb medical devices from 1 July 2026, you can add UDI labels your devices and submit UDI data to the AusUDID before this date.
If you choose to voluntarily comply with the UDI requirements, we recommend you meet all requirements. This will reduce confusion for end users such as healthcare professionals, hospitals and patients.
If you have large numbers of UDI records or intend to use one of the Machine to Machine submission methods available, we urge you to prepare your UDI records well in advance of to the UDI compliance start date for your device(s).
If you are using the Machine to Machine HL7 SPL submission method, you must complete testing in our testing database prior to submission in the live database.
If you are using the Machine to Machine National Product Catalogue (NPC) submission method, you must ensure your data in the NPC is up to date and ready for submission.
If you are submitting UDI records using the Online Portal or Bulk Upload Microsoft Excel template, we recommend testing the functionality and ensuring your data sets are complete.
Further information on specific AusUDID submission methods is available on the UDI Hub.
If you are unable to submit UDI records within 30 days of the UDI compliance start date for your devices(s) due to not having prepared your UDI records or tested your data submission methods, you will be considered non-compliant (see below for information on non-compliance).
UDI compliance start dates
The UDI compliance start date refers to the date from which you must be compliant with the Australian UDI requirements for your device.
We are introducing UDI compliance in stages - with the compliance date depending on:
- the device risk class - high-risk devices must meet the UDI requirements earlier
- whether the device is supplied in Australia under European Medical Device Directive (EU MDD) or In Vitro Diagnostic Directive (EU IVDD) certificates - transitional arrangements are in place that align with the EU’s MDD to MDR transition
- whether it is an existing device - there are requirements for some devices that have been manufactured before the compliance date.
Non-compliance
As the UDI requirements, including UDI compliance start dates, form part of the Essential Principles, we may take regulatory action if you are non-compliant, including:
- suspension or cancellation of your devices from the Australian Register of Therapeutic Goods (ARTG)
- applying civil penalties as outlined in Part 4-11, Division 1 of the Act
- issuing infringement notices.
You can learn more about compliance actions and outcomes on the TGA website.
If you cannot meet the UDI requirements after the UDI compliance start date for your medical device, you may choose to submit an application for consent to supply. You must lodge the application well in advance of the compliance start date with supporting documentation. We must consider and make a decision on the application before you supply the non-compliant device.
Please note that a consent to supply application is considered by the delegate on a case-by-case basis and granted in exceptional circumstances for limited periods of time.
UDI compliance milestones and obligations
There are 2 major UDI compliance milestones for each device class. At each major compliance milestone, you have obligations you must meet under UDI requirements. These milestones are:
- Compliance with labelling requirements and submission of UDI records to the AusUDID
- Compliance with Direct Marking requirements and submission of Direct Marking information to the AusUDID.
Compliance for labelling and submitting data to the AusUDID
The first major compliance milestone is for UDI labelling and submitting UDI records.
To meet this milestone, you must:
- Apply the UDI Carrier to device labels and applicable higher levels of packaging
- Supply UDI-DIs and related information to the AusUDID (within 30 days of the device being next supplied in Australia)
- Link your ARTG ID to your UDI record(s)
- Ensure your UDI record contains data that is up to date while the device is in distribution
- Include the UDI on Patient Implant Cards for implantable devices
- Include the UDI on any notifications to the TGA, including adverse events, incident reports and market actions including recalls, alerts and corrections
- Ensure any additional device specific requirements are met.
For information on UDI labelling and UDI record submission requirements, see Complying with the Unique Device Identification requirements for medical devices.
Callum the sponsor
Callum supplies Class IIb medical devices. He must meet UDI requirements for these devices at the UDI compliance start date for Class IIb devices, which is 1 July 2026.
He has ensured his manufacturer has allocated a UDI for these devices, and the manufacturer has labelled the device and applicable levels of packaging.
Callum must supply the UDI data for this device to the TGA within 30 days of the device next being supplied in Australia after 1 July 2026. Callum must supply this data in the form of a UDI record in the AusUDID.
Callum is next supplying the device on 1 July 2026, so he must submit his UDI data to the AusUDID by 31 July 2026.
Callum submits his UDI record for this device on 1 July 2026 which ensures he is compliant with UDI requirements.
Callum and his manufacturer may also voluntarily choose to meet UDI requirements earlier. If his manufacturer chooses to voluntarily allocate and apply a UDI to these devices, we recommend Callum supply the data to the TGA in the form of a UDI record in the AusUDID when he first begins to supply these devices. This will reduce confusion for end users such as healthcare and patients by having UDI records that match the UDI on the devices’ labels.
Compliance with Direct Marking and Direct Marking Device Identifier (DI) submission to the AusUDID
The second major compliance milestone is for directly marking reusable devices and submitting the Direct Marking information to the AusUDID within your existing UDI records.
To meet this milestone, you must:
- Directly Mark your devices, if applicable
- Include the Direct Marking information on your UDI record.
For information on Direct Marking, see Complying with the Unique Device Identification requirements for medical devices.
If you do not supply reusable medical devices in Australia, this compliance milestone does not apply.
Medical device UDI compliance start dates
The UDI compliance start dates for each device class are outlined in the table below.
| Requirement | Class III | Class IIb | Class IIa | Class Is |
|---|---|---|---|---|
| Placing UDI Carrier on the label of device(s) | 1 July 2026 | 1 July 2026 | 1 July 2027 | 1 July 2028 |
| Submitting UDI-DIs and related data to the AusUDID | 1 July 2026 | 1 July 2026 | 1 July 2027 | 1 July 2028 |
| Direct Marking of the medical device and supplying Direct Marking DI to the AusUDID | 1 Jan 2028 | 1 Jan 2029* Not applicable for implantable medical devices. | 1 Jan 2029 | 1 Jan 2029 |
In vitro diagnostic (IVD) UDI compliance start dates
The UDI compliance start dates for each IVD device class are outlined in the table below.
| Requirement | Class 4 IVD | Class 3 IVD | Class 2 IVD | Class 1 IVD |
|---|---|---|---|---|
| Placing UDI Carrier on the label of IVD(s) | 1 July 2028 | 1 July 2028 | 1 July 2029 | 1 July 2029 |
| Submitting UDIs and related data to the AusUDID | 1 July 2028 | 1 July 2028 | 1 July 2029 | 1 July 2029 |
| Direct Marking of the IVD and supplying Direct Marking DI to the AusUDID | 1 July 2029 | 1 July 2029 | 1 July 2030 | 1 July 2030 |
Relabelling of existing medical devices
Class III and IIb medical devices that were manufactured and labelled prior to 1 July 2026 and are still within the sponsor’s control on or after 1 July 2029, must be relabelled with a UDI compliant label. The UDI record for these devices must also be submitted to the AusUDID, if not previously submitted.
You are only required to meet UDI requirements for existing Class III and Class IIb medical devices.
All other device classes manufactured or labelled prior to the respective UDI compliance date are exempt for the lifetime of the device.
Existing devices within the sponsor’s control
As illustrated in the following diagram, within the sponsor’s control means devices that have left the manufacturer but not yet supplied or distributed.
Figure 2: Explanation of existing devices within sponsor control
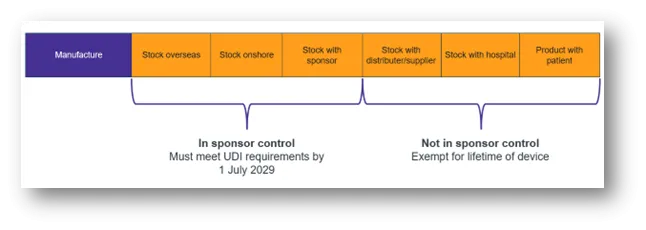
In sponsor control (must meet UDI requirements by 1 July 2029):
- Stock overseas
- Stock onshore
- Stock with sponsor
Not in sponsor control (exempt for lifetime of device)
- Stock distributer/supplier
- Stock with hospital
- Product with patient
Devices that are not in the sponsor’s control are exempt from UDI requirements for the lifetime of the device.
Geordie the sponsor
Geordie supplies Class III medical devices in Australia. Geordie has 1000 of his Class III devices in his local warehouse and 1000 of these devices already supplied in a hospital.
By 1 July 2026, Geordie’s manufacturer is labelling newly manufactured devices with UDI compliant labels and Geordie has submitted the UDI records to the AusUDID.
If Geordie still has the 1000 devices in his warehouse by 1 July 2029, he must relabel these devices with a UDI and supply the UDI record(s) to the AusUDID (if the UDI data is different to what he previously entered in the AusUDID).
If Geordie has already supplied these 1000 devices to a hospital, distributor, supplier or patient, these devices are exempt from the UDI requirements for their lifetime.
Devices that Geordie has already supplied to the hospital are exempt for the lifetime of the device and Geordie is not required to meet UDI requirements for these devices.
All the devices that Geordie supplies that are manufactured after 1 July 2026 must meet UDI requirements.
Geordie the sponsor
Geordie also supplies Class Is medical devices in Australia.
Because the existing devices requirements only apply to Class III and Class IIb devices, Geordie does not need to relabel devices that were manufactured or labelled before the UDI compliance start date for Class Is medical devices.
However, all Class Is medical devices manufactured after the UDI compliance start date for Class Is devices must meet UDI requirements. This means any devices that are manufactured after 1 July 2028, these devices must meet UDI requirements.
Geordie can continue to supply both batches of devices until the supply of existing devices is exhausted.
Relabelled existing devices
If you relabel your medical device or IVD after the UDI compliance start date passes, you must meet all the UDI requirements for this newly labelled device. This includes submitting UDI data to the AusUDID.
Relabelling your device after the UDI compliance start date does not impact the exemption from Direct Marking requirements. Your device remains exempt from Direct Marking requirements for the lifetime of the device.
Refurbished existing devices
Refurbishment is defined as a substantial rebuild from one or more used medical devices that may render it a ‘new’ medical device under the Regulations.
If you refurbish an already used medical device, you are considered the manufacturer of the device.
Your responsibilities as a manufacturer of a refurbished device depend on whether or not you are also the original manufacturer of the device.
Refurbishing a device originally manufactured by different manufacturer
If you refurbish a device that was originally manufactured by different manufacturer, you are considered the manufacturer of the refurbished device. In this scenario, you are required to allocate a UDI-DI to the refurbished device. You must meet all relevant UDI requirements for this device. Your device also requires an ARTG inclusion. These devices are not considered existing devices and must meet UDI requirements at the relevant UDI compliance start date.
Refurbishing your own device
If you refurbish a device that you originally manufactured, you may be able to resupply this device under the existing ARTG, where:
- the refurbishment has not changed its intended purpose
- the device is considered the same model of device.
If you have changed the intended purpose of the device when refurbishing the device, this is considered a new device and is no longer an existing device. You must meet UDI requirements at the relevant UDI compliance start date.
If the device is no longer considered to be within the set limits of specifications, performance, size and composition of the original model of device, it is considered a new model of device. In this case, you must meet UDI requirements by the relevant UDI compliance start date.
If you refurbish a device that you originally manufactured, however this device does not yet have a UDI-DI allocated to it, you must meet UDI requirements by the UDI compliance start date. For example, if the device was previously not required to meet requirements as it was not mandatory at the time of manufacture, however the device is now in scope of UDI requirements at the time of remanufacture, you must meet UDI requirements for this device.
Refurbished devices that are refurbished after the UDI compliance start date must meet the Direct Marking requirements, if applicable. Refurbished devices are not exempt from Direct Marking.
Angus the refurbisher
Angus refurbished a Class Is medical device that he originally manufactured to supply for reuse. In the process, Angus:
- stripped the device
- checked the components and replaced components not suitable for re-use
- assembled the device and tested the device against the original specifications of the device
- identified the device as a refurbished device.
Angus has certified the device is suitable for reuse. He retains the legal liability for the quality, safety and performance of the device. Since he did these activities, Angus remains the manufacturer of the device. This device did not change intended purpose and is considered the same model of device.
Angus refurbished these devices after 1 July 2028 so Angus must now meet UDI requirements, as this device is no longer considered an existing device. This includes Angus allocating and applying the UDI to the device.
As Angus is also the sponsor of the device, he must meet the obligations of a sponsor. These include submitting the UDI-DI and related data to the AusUDID and linking the relevant ARTG to the UDI record within 30 days of supply of the device.
Further information on refurbished devices is available in Complying with the Unique Device Identification requirements for medical devices.
Alignment with EU MDR and IVDR transition timeframes
The EU MDR transition extension introduces additional transition provisions for devices with an extension to their EU MDD certificate.
As devices with the extended MDD certificates will not be UDI compliant in the EU until they are MDR compliant, we are providing transitional arrangements that align with MDD extension dates.
Devices supplied in Australia under an EU MDR certificate must meet the Australian UDI compliance start dates. Devices supplied in Australia under any other overseas regulator conformity assessment certificate must meet the Australian UDI compliance start dates.
Transitional dates for devices supplied under MDD and IVDD
The tables below summarise the transitional dates for UDI compliance for devices you supply in Australia under an EU certificate.
Medical Devices
| Requirement | Class III | Class IIb implantables | Class IIb non-implantables | Class IIa | Class Is |
|---|---|---|---|---|---|
| Placing UDI Carrier on the label of device(s) | 1 Jan 2028 | 1 Jan 2028 | 1 Jan 2029 | 1 Jan 2029 | 1 Jan 2029 |
| Submitting UDI-DIs and related data to the AusUDID | 1 Jan 2028 | 1 Jan 2028 | 1 Jan 2029 | 1 Jan 2029 | 1 Jan 2029 |
| Direct Marking of the medical device and supplying Direct Marking DI to the AusUDID | 1 Jan 2028 | Not applicable | 1 Jan 2029 | 1 Jan 2029 | 1 Jan 2029 |
IVDs
| Requirement | Class 4 | Class 3 | Class 2 | Class 1 |
|---|---|---|---|---|
| Placing UDI Carrier on the label of IVD(s) | 1 July 2028 | 1 Jan 2029 | 1 Jan 2030 | 1 Jan 2030 |
| Submitting UDI-DIs and related data to the AusUDID | 1 July 2028 | 1 Jan 2029 | 1 Jan 2030 | 1 Jan 2030 |
| Direct Marking of the medical device and supplying Direct Marking DI to the AusUDID | 1 July 2029 | 1 July 2029 | 1 July 2030 | 1 July 2030 |
If the devices you supply in Australia are transitioned to an EU MDR certificate before the transitional dates for EU MDD certificates, and after the Australian UDI compliance start dates, you must meet UDI requirements for these devices at the time of transition.
We may contact sponsors at any time to ascertain the state of their devices, as part of our ongoing compliance activities.
Charlotte the sponsor
Charlotte supplies a Class III medical device in Europe. These devices are supplied in Europe under an MDD certificate.
Charlotte also supplies these devices in Australia under an MDD certificate. Charlotte’s devices require transitional arrangements and are not UDI compliant.
Under UDI compliance start dates, Charlotte’s Class III devices must be compliant by 1 July 2026. However, with the transitional dates for devices supplied under an EU certificate, Charlotte has an extension until 1 January 2028 to meet UDI requirements.
Charlotte does not need Consent to Supply her Class III devices during this time.
If Charlotte cannot meet UDI requirements by 1 Jan 2028, she must apply for Consent to Supply.
If her devices become MDR certified before 1 Jan 2028, Charlotte must meet UDI requirements in Australia. This includes ensuring that her devices labels bear a UDI Carrier, and her UDI-DIs and related data are supplied to the AusUDID.
UDI requirements for new applications under an MDD certificate
For new Applications for Inclusion that rely on an MDD certificate, submitted after the expiry date listed on the certificate, the sponsor must provide evidence that the manufacturer is eligible for extended validity under the EU MDR. This will form part of the Application for Inclusion process.
The UDI MDR transitional arrangements will apply for the devices supplied under this new application.
Lucy the sponsor
Lucy supplies a Class IIa medical device in Europe. These devices are supplied in Europe under a MDD certificate and are ‘legacy’ devices. Because of this, Lucy has an extension until 31 December 2028 to meet UDI requirements for this Class IIa device in the EU.
Lucy applies to the TGA to supply these devices in Australia.
To minimise burden on sponsors and ensure quality data is provided to the AusUDID, the TGA will recognise the extension provided in the EU. For Lucy, this means her Class IIa legacy device has until 1 Jan 2029 to be UDI compliant in Australia.
When Lucy’s device becomes MDR compliant (including UDI compliant), and Lucy supplies the MDR compliant devices in Australia, Lucy must meet Australian UDI requirements by the UDI compliance start date for MDR compliant devices. For Class IIa devices, this is 1 July 2027.
If Lucy’s device is not UDI compliant by the UDI compliance start date, Lucy can apply for Consent to Supply.
UDI requirements for devices supplied under an MDR certificate
You must meet UDI requirements for devices that are supported by an EU MDR certificate from the time of the UDI compliance start date for the device’s risk class This includes new and existing applications.
Hailey the sponsor
Hailey supplies a Class Is medical device in Europe and Australia. These devices are supplied in Europe and Australia under MDR certification. These devices do not require transitional arrangements and are UDI compliant.
As the devices are MDR compliant, Hailey must meet Australian UDI requirements for the Class Is devices by the Australian UDI compliance start date for MDR compliant Class Is devices, which is 1 July 2028.
This includes Hailey’s devices requiring a UDI on the label and Hailey supplying the UDI and related data to the AusUDID.
Hailey may also choose to meet UDI requirements prior to 1 July 2028.
Appendix A - Detailed implementation timelines
Detailed implementation timelines are provided below for both medical devices and IVDs.
The milestones shown on these timelines are:
- Start - the start date from which UDI compliance with UDI requirements becomes mandatory, which includes labelling and submission of UDI records to the AusUDID
- Direct Marking - the date from which direct marking and submission of direct marking information to the AusUDID must occur (where applicable)
- MDD Devices Transitioned - the date from which all device(s) supplied under MDD certificates must be UDI-compliant for labelling and UDI record(s) must be submitted to the AusUDID
- Existing Device Relabelled - the date from which Class III and Class IIb existing device(s) must be UDI compliant for labelling and UDI record(s) must be submitted to the AusUDID.
Medical Devices detailed implementation timeline
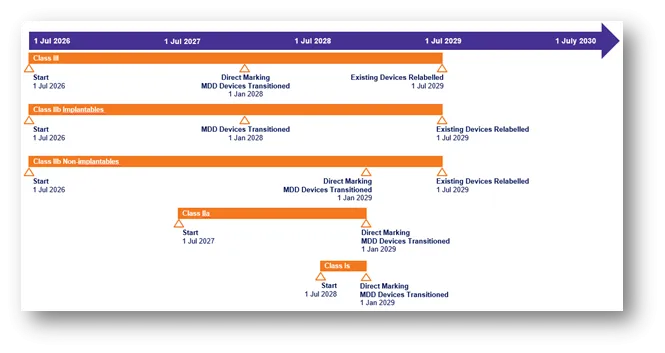
Class III
- Start: 1 July 2026
- Direct Marking and MDD devices transitioned: 1 January 2028
- Existing devices relabelled: 1 July 2029
Class IIb implantables
- Start: 1 July 2026
- MDD devices transitioned: 1 January 2028
- Existing devices relabelled: 1 July 2029
Class IIb non-implantables
- Start: 1 July 2026
- Direct Marking and MDD devices transitioned: 1 January 2029
- Existing devices relabelled: 1 July 2029
Class IIa
- Start: 1 July 2027
- Direct Marking and MDD devices transitioned: 1 January 2029
Class Is
- Start: 1 July 2028
- Direct Marking and MDD devices transitioned: 1 January 2029
IVD detailed implementation timeline
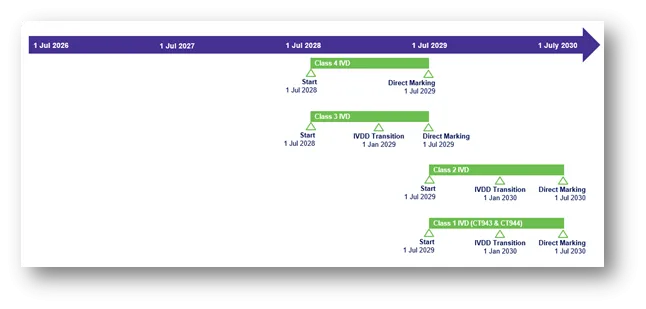
Class 4 IVD
- Start: 1 July 2028
- Direct Marking: 1 July 2029
Class 3 IVD
- Start: 1 July 2028
- IVDD transition: 1 January 2029
- Direct Marking: 1 July 2029
Class 2 IVD
- Start: 1 July 2029
- IVDD transition: 1 January 2030
- Direct Marking: 1 July 2030
Class 1 IVD (CT943 and CT944)
- Start: 1 July 2029
- IVDD transition: 1 January 2030
- Direct Marking: 1 July 2030

