GMP clearance Sponsor Information Dashboard (SID)
SID provides industry with regular updates about the backlog reduction, actual processing times, existing workloads, requesting prioritisation and other key messages for GMP clearance applications.
The backlog of Good Manufacturing Practice (GMP) Clearance Compliance Verification (CV) applications is continuing to reduce.
All backlog reduction strategies have been implemented and a recap has been published.
The below data is from 1 March 2026 and will be updated every 4 to 6 weeks to ensure it remains current.
Backlog reduction progress
The graphs below illustrate the trend of GMP Clearance applications over time. Further details on actual numbers can be read from opening the drop-down menu under each graph.
Figure 1 shows the trend of the total number of CV applications in our lodgement queue over the past 12 months. The total number of CV applications steadily reduced in 2025, with a steep drop at the beginning of Q3 2025 due to the backlog reduction strategy. Since the backlog reduction strategy was introduced on 1 July 2025, the rate of reduction has also increased as expected.
Following the holiday period, reduction rates have returned to the levels seen in late 2025.
Figure 1 - total number of CV applications pending assessment between 1 March 2025 and 1 March 2026
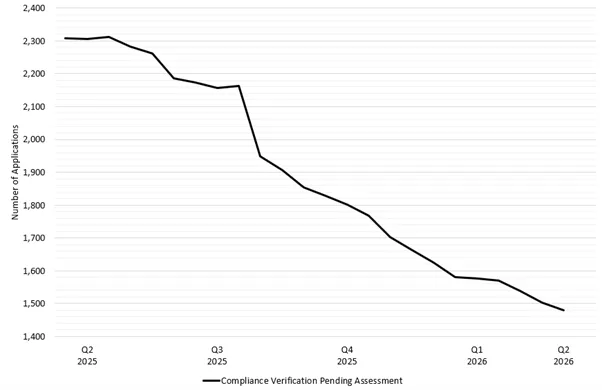
Figure 1 shows a line graph detailing the total number of Good Manufacturing Practice (GMP) Compliance Verification (CV) clearance applications between the end of Q1 2025 and Q2 2026.
The vertical axis represents the total number of GMP clearance applications within the TGA’s application lodgement system.
The horizontal axis represents the date broken down by quarter and year.
Starting at ~2300 applications at the end of Q1 2025, the line steadily declines, reaching ~1480 applications by 1 March 2026. This equates to a reduction of ~35%
Figure 2 shows the trend of applications in our CV streams over the past year with each line representing a different stream.
Both the Non-Sterile Active Pharmaceutical Ingredient (NS-API) and Non-Sterile Finished Product (NS-FP) streams are reducing as expected whilst both sterile streams continue to remain relatively steady.
Figure 2 - number of CV applications in lodgement by stream between 1 March 2025 and 1 March 2026
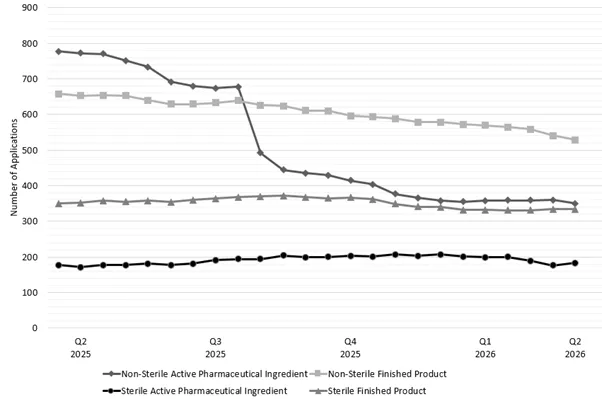
Figure 2 shows a line graph detailing the number of Good Manufacturing Practice (GMP) clearance compliance verification applications pending assessment in each stream between the end of Q1 2025 and Q2 2026.
The vertical axis represents the total number of GMP clearance applications within the TGA’s application lodgement system.
The horizontal axis represents the date broken down by quarter and year.
There are four lines represented within the graph.
The line with a diamond marker at the top of the graph represents non-sterile active pharmaceutical ingredient compliance verification applications. This shows a sustained decrease in application numbers from ~840 to ~680 by the end of June 2025 followed by a sharp decrease to ~500 by August 2025. These numbers further decrease to ~350 by 1 March 2026.
The next line with a square marker represents non-sterile finished product compliance verification applications. The line shows a consistent decrease in application numbers from ~650 in Q2 2025 to ~530 by 1 March 2026.
The third line with a triangle marker represents sterile finished product compliance verification applications. This shows that there was a slight increase in application numbers from ~350 to ~380 between Q1 2025 and Q3 2025 but have decreased back to ~335 by 1 March 2026.
The bottom line with a circle marker represents sterile active pharmaceutical ingredient compliance verification applications. This shows that application numbers have remained relatively steady between ~190 and ~200 within the last year.
GMP clearance workload volumes
Figure 3 shows a snapshot of all GMP Clearance applications in the system at different stages of evaluation or queue categories. The largest number are 'complete' applications in lodgement queues ready for evaluation (This includes both MRA and CV application types). The total number of all applications on 1 March 2026 was 1647.
Figure 3 - total number of GMP clearance applications and the applications status as of 1 March 2026

Figure 3 shows a bar graph detailing total numbers of Good Manufacturing Practice (GMP) clearance applications and the applications status.
The vertical axis represents the number of applications.
The horizontal axis represents the status of the applications.
There are nine bars:
The first bar from the left side of the graph represents complete applications awaiting assessment, there are 845 applications represented. This is reduced from 916 in February. with more applications being picked up for evaluation.
The second bar represents assessments in progress, that is applications currently being considered by evaluators, there are 285 applications represented. This has decreased slightly from 303 in February.
The third bar represents administrative applications awaiting assessment, this includes updates for manufacturer names and addresses as well as other application types, there are 147 applications represented, an increase of 1 since February.
The fourth bar represents incomplete applications, there are 73 applications represented, a decrease of 3 since February.
The fifth bar represents applications awaiting payment, there are 73 applications represented, an increase of 8 since February.
The sixth bar represents applications awaiting receipt there are 64 applications represented, an increase of 16 since February.
The seventh bar represents letter of access applications awaiting assessment, there are 60 applications represented, an increase of 2 since February.
The eighth bar represents extension applications, there are 53 applications represented, a decrease of 57 since February.
The ninth bar on the far right of the graph represents applications which are currently on hold (typically for GMP non-compliance investigations), there are 47 applications represented, an increase of 4 since February.
GMP clearance current processing timeframes - rolling 3 monthly data
Figure 4 shows the current percentile range for actual processing times for each CV stream, measured in TGA working days. Each bar is divided into percentiles, which represent the percentage of applications completed within a certain number of days. For example, the 50th percentile means half of the applications were completed in that time or less.
The percentiles build on each other - for example, the 50% mark includes all applications completed within the 10% and 25% ranges as well. It is expected these percentiles will continue to fluctuate as we complete older applications from the system and manage competing priorities. As we return to a steady state, requests for prioritisation should reduce and the overall timeframes should also reduce.
Due to the backlog reduction strategy and prioritisation requests, we have purposefully not been actioning certain applications that are renewals as the original GMP Clearances were extended. This has allowed ongoing supply whilst we focused on applications linked to new market access or critical variations.
As we begin processing these older applications, this is affecting the below data. We will be providing alternative data-sets in future updates until such time that more useful timeframe data becomes available.
Figure 4 - percentages of CV applications completed within TGA working days
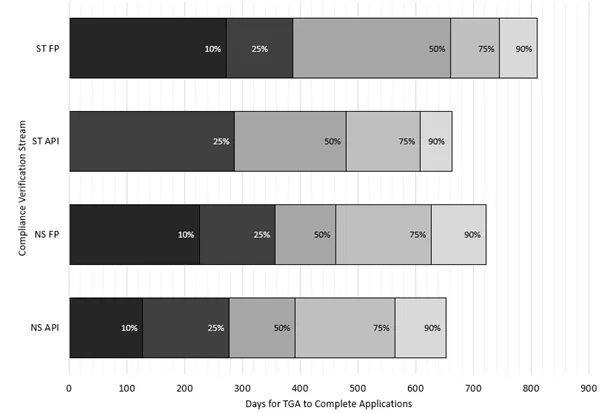
ST FP: sterile finished product applications
ST API: sterile active pharmaceutical ingredient applications
NS FP: non-sterile finished product applications
NS API: non-sterile active pharmaceutical ingredient applications
Figure 4 shows a bar graph detailing percentages of Good Manufacturing Practice (GMP) clearance compliance verification applications completed within specified TGA working days.
The vertical axis represents the compliance verification application streams.
The horizontal axis represents the number of days taken by the TGA to complete applications.
There are four bars, the first bar from the bottom of the graph represents non-sterile active pharmaceutical ingredient compliance verification applications. This shows that:
- ten percent of applications are completed within 128 days,
- twenty five percent of applications are completed within 278 days,
- fifty percent of applications are completed within 392 days,
- seventy five percent of applications are completed within 564 days
- ninety percent of applications are completed within 653 days.
The second bar represents non-sterile finished product compliance verification applications. This shows that:
- ten percent of applications are completed within 226 days
- twenty five percent of applications are completed within 357days
- fifty percent of applications are completed within 462 days
- seventy five percent of applications are completed within 627 days
- ninety percent of applications are completed within 722 days
The third bar represents sterile active pharmaceutical ingredient compliance verification applications. This shows that:
- ten percent of applications are completed within 1 day
- twenty five percent of applications are completed within 287 days
- fifty percent of applications are completed within 480 days
- seventy five percent of applications are completed within 608 days
- ninety percent of applications are completed within 663 days
The fourth bar represents sterile finished product compliance verification applications. This shows that:
- ten percent of applications are completed within 272 days
- twenty five percent of applications are completed within 387 days
- fifty percent of applications are completed within 661 days
- seventy five percent of applications are completed within 746 days
- ninety percent of applications are completed within 811 days
GMP clearance target processing timeframes - rolling 3 monthly data
The figures below provide an overview of the target and actual processing times for certain application types, measured in TGA working days. The actual processing time applies for 90 percent of applications completed within the last 3 months.
-
30
Target MRA application processing time
-
20
Actual MRA application processing time
MRA applications have been consistently processed below our target processing time. Due to the rolling nature of the SID data and the lag this creates, the actual processing times have only now reduced to approximately 20 TGA working days.
Application Quality - rolling 6-monthly data snapshot
The figures below provide an overview of how many applications required a Request For Information (RFI) or were incomplete at the time of submission.
-
11 % of 387 applications
Percentage of Mutual Recognition Agreement (MRA) applications requiring an RFI
-
35 % of 701 applications
Percentage of incomplete CV applications
-
68 % of 701 applications
Percentage of Compliance Verification (CV) applications requiring an RFI
-
32 % of 79 applications
Percentage of TGA certificate applications requiring an RFI
-
76 % of 33 applications
Percentage of applications using a letter of access (LoA) requiring an RFI
Prioritisation requests
We will continue to process effective applications as quickly as possible. Medicine shortages remain a high priority for us, and we will continue to process these when sponsors have provided the required information.
Please note that we will liaise internally with the medicine shortages team to verify requests and seek additional information such as supply and market data.
For any request for prioritisation, you must provide the required information outlined below via email:
- The product name and existing ARTG number (where applicable)
- The category of medicine (Complementary, Non-prescription, Prescription etc.)
- The product submission type and submission number and any applicable milestone dates (for example, priority review pathway or NCE, PM-XXXX-XXXXX-X-X etc.)
- For variations, you need to provide information about the change as certain changes made by manufacturers may require additional GMP evaluation.
- For requests linked to reportable medicines, the medicines shortage notification number (for example MS-XXXX-XX-XXXXX-X)
- Any other information to help us understand the urgency of the situation. For example, existing stock levels or run-out dates, negative effect on patients or business etc.
Featured notices
-
News articlesA review of the GMP Clearance backlog reduction strategies that have been implemented and ongoing monitoring and reporting communication.
-
News articlesThe GMP Clearance SID now contains additional data as well as progress information on the Compliance Verification (CV) backlog reduction strategy.
-
News articlesInformation on the completion of automatic extensions for Good Manufacturing Practice (GMP) clearances and next steps.
Related resources
-
PageFind out about obtaining Good Manufacturing Practice (GMP) clearance for an overseas manufacturing site.
-
User guideThis user guide is for sponsors wanting to submit GMP clearance application by the TGA Business services (TBS) portal.
-
User guideCheck out our user guide to assist sponsors when using the code tables for GMP Clearance applications.

