How to apply for consent to supply for medical devices that do not meet the Essential Principles
To help sponsors apply for permission to import, supply, or export medical devices that do not fully comply with one or more Essential Principles for a limited period.
To guide sponsors through applying for consent to import, supply or export medical devices that do not meet one or more parts of the Essential Principles (EPs) for a limited period.
Note
Each application for consent needs to be assessed by us. Application submission does not guarantee consent will be granted.
Background
Under sections 41MA and 41MAA of the Therapeutic Goods Act 1989 (the Act), it is an offence to import, supply or export medical devices that do not meet one or more of the Essential Principles for safety and performance.
In some circumstances, we may grant consent to supply for a defined period when compliance is temporarily not possible, provided that any safety or performance issues are appropriately mitigated and the benefits of supply outweigh the risks.
This helps maintain access to critical devices without compromising patient safety.
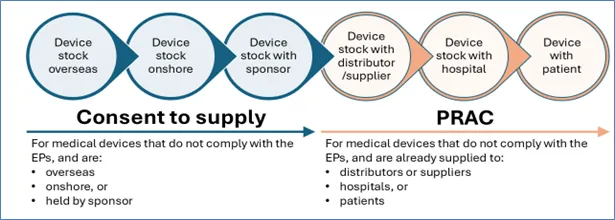
Consent is required before supplying non-compliant device stock that is:
- overseas
- onshore, or
- held by the sponsor.
Consent is not required for non-compliant device stock already supplied to:
- distributors or suppliers
- hospitals, or
- patients.
Applying for consent to supply
Any sponsor of medical devices can apply for consent to import, supply or export.
This includes sponsors of exempt goods such as vaping devices, sponsors with pre‑market applications (applications for inclusion), and sponsors with devices already included in the Australian Register of Therapeutic Goods (ARTG).
Applications for inclusion in the ARTG under the consent to supply pathway will only be approved in exceptional circumstances.
Approval will only proceed where there are no concerns about the safety or performance of the device.
What consent to supply covers
Consent to supply covers medical devices, including IVDs and exempt devices (e.g. vaping devices), that do not meet one or more Essential Principles for safety and performance for a defined period.
What consent to supply does not cover
Consent to supply does not cover:
- permanent supply of non-compliant devices,
- non-compliant devices already in the market,
- devices where risks to patient safety cannot be adequately mitigated,
- reducing business costs, such as over labelling,
- lapsed or expired conformity assessment certification,
- medicines, including vaping substances such as nicotine or cannabis (these require separate applications under sections 14 and 14A of the Act).
Consent to supply
Market action and consent to supply processes
The Procedure for recalls, product alerts and product corrections (PRAC) market action process and the consent to supply processes usually occur at different stages in the supply chain, although there are some circumstances where both processes may need to be applied concurrently.
For example:
- a device defect is found after supply has occurred → requiring a market action (recall, product alert or product correction), and
- at the same time, the same sponsor needs to maintain supply whilst fixing the device defect which is in breach of one or more Essential Principles → requiring a consent to supply application for any remaining stock in their possession or for future batches.
Both processes aim to protect patient safety while maintaining continuity of supply. Sponsors must keep clear records and communicate with us throughout these processes.
Checklist for sponsors
Before acting, confirm:
-
Has the product already been supplied?
- If yes → consider a market action (recall, product alert, or product correction)
- If no → consider consent to supply if non-compliance is unavoidable
-
Is there a significant or imminent risk to patient safety?
- If yes → perform an immediate market action and cease supply
-
Is the non-compliance temporary and justified?
- If yes → apply for consent to supply with supporting evidence and a risk mitigation plan
-
Have you documented all actions and notified us?
- Maintain clear records for both market action and consent to supply processes
Applying for consent to supply via the TGA Business Services portal
Submit your application through the TGA Business Services (TBS) portal.
The updated online application form supports devices not meeting the Essential Principles, exempt goods such as vaping devices, devices with pre‑market applications (applications for inclusion), and devices already in the ARTG.
The unique identifier generated upon submission is for our tracking only and must not be used for marketing.
The online form is not for medicines such as vaping substances (e.g., nicotine or cannabis).
Information to include with your consent application
- identify which Essential Principles have not been met
- supply numbers impacted (include batch/lot numbers where relevant)
- the consent period (from‑date and to‑date) during which devices will not meet the Essential Principles
- circumstances explaining why the devices do not meet the Essential Principles
- advise whether market action notifications for the non-compliant devices have been, or will be, submitted to the TGA, or have been recently agreed
- potential for supply shortage if consent is not granted (immediate and future impacts)
- risks associated with not meeting the Essential Principles
- risk mitigation strategies during the consent period
- strategies and timeframe to restore compliance with the Essential Principles
Legislative requirements and review timeline
Under section 41MA (criminal offences) and section 41MAA (civil penalties) of the Act, supplying without consent is unlawful.
There are no legislated timelines for reviewing consent to supply applications under sections 41MA and 41MAA of the Act.
Review time depends on the quality and completeness of the application. If information is insufficient, the timeline will be extended.
Sponsors must provide all information required to satisfy us that consent should be granted.
Examples of when to apply for consent
Example 1
A sponsor has a medical device included in the ARTG that does not comply with Essential Principles 1, 2, 3 and 4, which relate to:
- Essential Principle 1 – Use of medical devices not to comprise health and safety
- Essential Principle 2 – Design and construction of medical devices to conform with safety principles
- Essential Principle 3 – Medical devices to be suitable for intended purpose
- Essential Principle 4 – Long term safety
The non-compliance means the device is defective and poses a significant risk of harm to patients. Under normal circumstances, supply should cease immediately until compliant or non-defective devices are available.
Key points
- The device fails to meet multiple Essential Principles critical to safety and performance.
- Continuing supply without corrective action would present a serious risk to patient health.
- Supply of the device should stop until compliance is achieved.
When consent may be considered
If there is a critical shortage and withholding the device would result in imminent death or severe deterioration of patient health, the sponsor may seek consent to supply under the Act.
Required actions
The sponsor should:
- Submit an application for consent to supply via the TGA Business Services portal.
- Demonstrate that the device is essential for life-saving treatment and that no alternatives are available.
- Provide risk mitigation measures, for example:
- Detailed warnings and instructions to clinicians.
- Limit supply to controlled environments (e.g., tertiary hospitals).
- Implement enhanced monitoring and reporting of adverse events.
- Timelines for achieving compliance.
- Pay the applicable fee for each ARTG entry.
Conditions of consent
If granted, consent:
- Will apply for a limited period.
- May include conditions such as specifying the exact number of devices to be supplied to designated tertiary hospitals.
If consent is not granted, the device cannot be imported, supplied or exported.
Example 2
A sponsor has a medical device included in the ARTG that does not comply with Essential Principle 13.4 because the Instructions for Use (IFU) have not been updated to reflect recent regulatory changes. The manufacturer is revising the IFU but cannot complete the update before the device needs to be supplied to maintain continuity of patient care.
Key points
- The device does not meet Essential Principle 13.4 related to safety and performance information.
- Full compliance is temporarily not achievable due to delays in producing and distributing the updated IFU.
- The sponsor intends to import and supply the non-compliant device stock within Australia.
Required actions
The sponsor should:
- Submit an application for consent to supply via the TGA Business Services portal.
- Provide a clear justification, including:
- Risk mitigation measures (e.g., interim instructions or supplementary notices).
- Timelines for achieving compliance.
- Pay the application fee.
Conditions of consent
If granted, consent:
- Will apply for a limited period.
- May include conditions such as providing the updated IFU by a specified date.
If consent is not granted, the device cannot be imported, supplied or exported.
Example of when not to apply for consent
Example 3
A sponsor has a medical device included in the ARTG where the current labels do not fully comply with the requirements of Essential Principle 13.1. For example, the labels omit a recently mandated warning statement. The sponsor can achieve compliance before supply by applying an over-label that includes the missing information.
Key points
- The device will comply with the requirements of Essential Principle 13.1 at the time of supply because the over-labelling process corrects the non-compliance.
- There is no period during which the device is supplied in a non-compliant state.
- Therefore, the sponsor does not need to apply for consent under section 41MA or section 41MAA, as the Act only requires consent when a non-compliant device is supplied.
Important considerations
- The over-label must be applied before the device is imported, supplied to the Australian market or exported from Australia.
- The sponsor should maintain records demonstrating that all supplied devices were corrected before distribution.
- If the sponsor cannot guarantee compliance, then consent would be required.
Example 4
A sponsor has a medical device included in the ARTG where the conformity assessment certification (manufacturer’s evidence) has lapsed. The device continues to meet all safety and performance requirements under the Essential Principles.
Key Points
- The device remains compliant with the Essential Principles.
- The issue relates to the absence of a valid conformity assessment certification.
- Sections 41MA and 41MAA of the Act do not apply, as these provisions only cover supply of devices that fail to meet one or more of the Essential Principles.
Important considerations
- The sponsor can continue supplying devices if they were manufactured on or before the date the conformity assessment certification expired.
- The sponsor cannot supply devices manufactured after the certification expiry date.
Invoices
Invoices are typically issued within 5 TGA working days. If you do not receive an invoice, contact our Revenue Management at accountsrec@health.gov.au.
Consent applications are assessed after all relevant fees are paid.
Fees
Applications will only be assessed after the application fee has been paid in full.
The application fee is published on the Fees and charges page and in Schedule 5 – Fees, Part 1 – General of the Therapeutic Goods (Medical Devices) Regulations 2002.
Reduced fees apply to applications made solely in relation to: Essential Principle 13 (information supplied by the manufacturer) and Essential Principle 13A (patient information materials), as set out on the Fees and charges page.

