Explaining the Data Protection Scheme for assessed listed medicine
An overview of the Data Protection Scheme for assessed listed medicines, and the criteria that must be met for information to be considered protected under the Scheme.
Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance provides an overview of:
- the Data Protection Scheme for assessed listed medicines
- criteria that must be met for information to be considered protected under the Scheme.
Legislation
Overview of the Data Protection Scheme
The purpose of the Scheme is to incentivise innovation by protecting the results of investment in the development of new works and technology. The Scheme is designed to prevent competitor(s) from seeking market authorisation of generic forms of an assessed listed [L(A)] medicine by relying on clinical trials generated and used by the sponsor of the originator medicine to obtain market authorisation.
Further, the Scheme intends to provide industry with the means to finance further research and development activities by preventing others from capitalising on their investment and innovation.
About the Scheme
The Data Protection Scheme for L(A) medicines provide successful applicants (sponsors) with a 5-year protection period for the clinical trial efficacy information supporting their L(A) medicine starting from the date the medicine was included in the Australian Register of Therapeutic Goods (ARTG). The Scheme is enabled under section 26AF of the Therapeutic Goods Act 1989 (the Act).
The Scheme is consistent with the data protection mechanism for registered medicines (including registered complementary medicines) established under section 25A of the Act. Section 25A provides a 5-year data protection period for information submitted to the TGA in relation to an application to register therapeutic goods consisting of, or containing, a new active ingredient.
Assessed listed [L(A)] medicines
L(A) medicines:
- are listed in the ARTG under section 26AE of the Act
- must carry at least one intermediate-level indication[1]
- must be certified, under subsection 26AB(2) of the Act, to be eligible for listing and safe for the purposes for which they are to be used
- include ingredients that have been previously evaluated by the TGA and are listed in the Therapeutic Goods (Permissible Ingredients) Determination (Permissible Ingredients Determination).
Applications for assessed listed medicines are made under section 23B of the Act. There are three categories of application: L(A)1, L(A)2 and L(A)3. The increasing levels correspond to the increasing complexity of applications, and consequently, increasing data requirements, evaluation timeframes and fees. For more information refer to Assessed listed medicines evidence guidelines.
Data eligible for protection
The raw clinical trial efficacy data and full clinical trial study report (herein collectively referred to as the clinical trial efficacy information) generated from a clinical trial[2] that is used to support a new intermediate indication for existing ingredient(s)[3] in an L(A) medicine application are eligible for data protection.
Data protection will usually apply to medicines submitted through the L(A)3 application category. Data protection can also apply to medicines submitted through the L(A)2 (Comparable Overseas Bodies) application category or L(A)C2 (new indication) application category if clinical trial efficacy information is used to support a new intermediate indication and certain criteria, as set out in this guidance, are met.
Criteria for data protection
Data protection is dependent on whether the delegate of the Secretary has relied on specific clinical trial efficacy information within the application and will coincide with the decision to list the L(A) medicine. Briefly, this clinical trial information must:
- not be available in the public domain (noting that this excludes information contained in a clinical trial registry)
- contain a clinical trial registry number
- be for a new intermediate indication-active ingredient combination
- be owned by the sponsor.
Data protection period
If successful, clinical trial efficacy information supporting a new intermediate indication that has been derived from a clinical trial will be restricted. This means that access to use this information for another L(A) application will be restricted for a 5-year protection period.
The 5-year protection period will begin from the date the approved medicine is included in the ARTG and ends 5 calendar years later. For example, a protection period starting 1 July 2021 will end on 30 June 2026.
Use of the information during the 5-year protection period will be restricted to the person (sponsor) in relation to whom the existing medicine is listed [section 26AF(g) refers]. This sponsor must certify that they own the information and have the legal right to use the information submitted with their application (see The sponsor must own the information for more details). During the protection period this person can give the TGA permission in writing to use this restricted information to support evaluation of another L(A) medicine. Once the protection period has expired, other sponsors can use the information that was restricted to support evaluation of their applications.
New intermediate indications
Definition of an intermediate indication
In addition to being able to use low-level indications from a list of pre-approved indications for listed medicines, sponsors of L(A) medicines must apply for at least one intermediate indication [4]. Intermediate indications can be higher level and more specific than those permitted for standard listed medicines, and will be consistent with, and supported by, the sponsor’s own clinical trial efficacy information.
Intermediate indications relate to:
- a use of the medicine in preventing, curing or alleviating a disease, ailment, defect or injury in persons, other than a form of the disease, ailment, defect or injury that, under the Therapeutic Goods Advertising Code, is a serious form; or
- a use of the medicine in connection with alleviating a disease, ailment, defect or injury in persons, being a form of the disease, ailment, defect or injury that, under the Therapeutic Goods Advertising Code, is a serious form
Structure of an intermediate indication
Sponsors of L(A) medicines may submit their own intermediate indication(s), which should conform to the prescribed indication structure and be supported by appropriate information.
A valid indication must:
- describe the specific therapeutic use(s) of the medicine
- contain an action and target.
An indication may also contain qualifiers to specify the severity, population, and timeframes related to the therapeutic use, depending on the specificity of the information provided to support the indication.
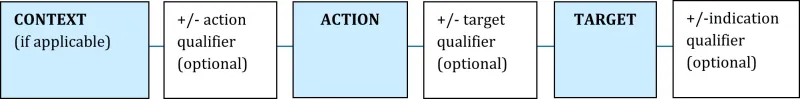
More information about how to structure indications for use in L(A) medicines can be found in the Assessed Listed Medicines Evidence guidelines.
When an intermediate indication is considered new
A new intermediate indication associated with an existing active ingredient must not be the same as an existing intermediate indication linked to the same ingredient. A new intermediate indication should clearly:
- define the target disease, ailment, defect or injury (including qualifiers if appropriate)
- define the action (and qualifiers if appropriate).
To be eligible for data protection:
- There must be no other L(A) medicine using the same intermediate indication-active ingredient(s) combination in the ARTG at the time the sponsor submits an L(A) medicine.
- An L(A) medicine with the same intermediate indication-active ingredient(s) combination must not have been previously included in the ARTG (i.e. was previously approved but has been cancelled).
Only the clinical trial efficacy information supporting the efficacy of a new intermediate indication-active ingredient(s) combination will be restricted.
An intermediate indication could be considered new if at least one of the core components-the action and/or target-and the intent and meaning, is different. For example, a new intermediate indication would differ from an existing intermediate indication if it contained:
- a change in action (e.g. change from alleviation to prevention of a non-serious form of a disease, ailment, defect or injury); and/or
- a different target (e.g. a different physiological factor or process; a different non-serious form of a disease, ailment, defect or injury; different symptoms); and/or
- a different target qualifier (e.g. a different target population).
How the Scheme works
Information must not be available to the public
This scheme does not place any restrictions on when a sponsor can publish their research work per se. Under this Scheme, only clinical trial efficacy information that is not available to the public can be restricted; however, this does not include information set out in a clinical trial registry prescribed in reg.15AA of the Regulations [s26AF(eb) refers].
Therefore, if a sponsor would like to restrict the clinical trial efficacy information supporting a novel intermediate indication-active ingredient(s) combination, then the sponsor cannot publish information pertaining to their clinical trial study until the L(A) medicine is included in the ARTG after receiving market approval.
Sponsors choosing to publish their work before the TGA has concluded its evaluation can do so. However, any information that becomes publicly available before the medicine is listed or during the assessment period (excepting that information set out in a clinical trial registry) will not be eligible for protection under this Scheme.
This means that other sponsors will be able to use this information to support their own applications. In this case, the TGA will not be in a position to prevent a competitor from submitting a literature-based submission for the same intermediate indication-active ingredient(s) combination that includes publicly available clinical trial efficacy information.
Once the clinical trial efficacy information is restricted, the sponsor can publish their results. See Sponsors must notify the TGA of publication details for more information about the sponsor's responsibilities when publishing their work.
Sponsors must provide raw clinical trial efficacy data and the full clinical trial study report
Sponsors will be required to provide the raw clinical trial efficacy data generated from a clinical trial supporting the efficacy of a new intermediate indication and the corresponding full clinical trial study report[5] (collectively referred to as the clinical trial efficacy information).
The sponsor must clearly identify the clinical trial efficacy information they would like to restrict. This is usually a pivotal clinical study (or studies) that is conducted on the product being applied for and may include bioequivalence, pharmacokinetic, or other clinical studies that the sponsor has relied on to support the efficacy of the medicine for the proposed new intermediate indication(s).
For the purpose of this Scheme, the biostatistics report forms an important component of the clinical trial study report. Considering that the unpublished clinical trial efficacy information includes raw data that has not been peer-reviewed, an independent biostatistics report or validation of the original biostatistics report should be provided with the application to allow the TGA to review and verify the presentation of the raw clinical trial information. The raw data alone, in the absence of the biostatistics report, does not provide sufficiently meaningful information for the evaluation.
The provision of raw clinical trial efficacy data and a full clinical trial study report is not a mandatory requirement if information supporting an L(A) medicine is not to be restricted under this Scheme. However, this information is required if sponsors wish to restrict eligible information.
The sponsor must own the information
Sponsors will need to certify that they own the clinical trial efficacy information to support the proposed new intermediate indication and have the legal right to use this information (see Appendix 1).
'Own' means that the sponsor has either conducted the study, or sponsored/cosponsored the study and has the legal right to use the study. Once the information is restricted, the sponsor can give the TGA permission to use their restricted information to support the evaluation of another L(A) medicine during the protection period.
If a sponsor's L(A) medicine application is successful, it is their responsibility to monitor any perceived breaches of the data protection and to pursue this with the alleged offender.
Note: the TGA will not be the arbiter, or manage issues, if a sponsor has submitted information that is not in the public domain and:
- they did not own the information; or
- did not have the legal right to use the information.
Sponsors applying for a new L(A) medicine, or to vary an existing L(A) medicine, will need to certify that they are not using another sponsor's restricted information, without permission, in their application. The TGA will rely on this certification. If, during assessment, the TGA finds that this certification is false or misleading (i.e. the sponsor has submitted or used restricted information without permission) then the application will lapse.
The TGA will publish a list of L(A) medicines with data protection for sponsors.
Sponsors must provide the unique clinical trial registry number
The clinical trial registry number is a registration number provided by a clinical trial registry when a sponsor registers their trial. Clinical trials may be included in more than one registry, and sometimes the information about a single trial entered into different registries may be slightly different even though the information in the individual registries is derived from the one trial. Therefore, if a clinical trial has been registered with more than one clinical trial registry, the sponsor will need to provide the TGA with all of the clinical trial registry numbers associated with the clinical trial.
The universal trial number (UTN)[6] must also be provided if available.
All clinical trial registry number(s) and the UTN (if available) will be included in the list of assessed listed medicines with data protection maintained by the TGA.
Sponsors must identify the new intermediate indication-active ingredient(s) combination relating to data protection
A clinical trial may support the efficacy of more than one new intermediate indication associated with a particular L(A) medicine. For example, a clinical trial might have multiple outcomes and may therefore demonstrate efficacy for more than one new intermediate indications.
Sponsors wishing to restrict clinical trial efficacy information supporting more than one intermediate indication-active ingredient(s) combination for the L(A) medicine must clearly identify each of the new intermediate indication-active ingredient(s) combinations.
Subsequent applications using the same intermediate indication-active ingredient(s) combination
Once a sponsor's L(A) medicine application is successful, the clinical trial efficacy information supporting the efficacy of a new intermediate indication-active ingredient(s) combination will be restricted for 5 years.
Other sponsors may submit an application to gain market approval for an L(A) medicine with the same intermediate indication-active ingredient(s) combination during this 5-year period if they can provide information (other than the restricted information) to support the efficacy of this combination.
However, this information (even if supported by an original clinical trial) will not be eligible for data protection as the first L(A) medicine (with the same intermediate indication-active ingredient(s) combination and associated data protection) has already been included in the ARTG [sections 26AF(2)(d)(ii) and (da) refers].
Sponsors should also note the following information as to when active ingredients are considered to be the same or different ingredients:
- Different salts with the same active component are considered to be different ingredients if they have their own entry on the Permissible Ingredients Determination, e.g. MgOH and MgSO4 are considered to be two separate ingredients.
- The same ingredient manufactured by separate processes is not considered to be two separate ingredients, i.e. two different solvent extraction processes of the same herb/herbal mix are not considered to be two distinct ingredients within the Permissible Ingredients Determination.
Sponsors must notify the TGA of publication details
Pre-publication
Once a sponsor's L(A) medicine has gained market approval and is listed in the ARTG, the sponsor will be able to publish the results of their clinical trial studies. It is important the clinical trial registry number(s) be included in the published article(s), because all information within the published article will be classified as restricted information by virtue of the clinical trial registry number(s).
Publication can only occur after:
- the L(A) medicine has received market approval and been listed in the ARTG; and
- the L(A) medicine and the respective clinical trial number has been included in the TGA's list of L(A) medicines with data protection.
Post-publication
Sponsors should notify the TGA when their article has been accepted for publication. Ideally, this should be done prior to publication (either in electronic or print format) so that the TGA can list the publication details in the list of L(A) medicines with data protection. This will make it clear to other sponsors that this information is restricted and will also help to prevent other sponsors from using this information to support another L(A) application.
Sponsors will be required to provide the TGA with the following details:
- article title
- author list
- journal title
- journal volume and pages (if available)
- the digital object identifier (if available).
It is the sponsor's responsibility to provide the TGA with any updates as information becomes available.
The TGA's responsibilities
The TGA will assess the information provided in an application within the legislated timeframes. When making the decision to grant market approval, the TGA must have relied on the submitted clinical trial efficacy information for that information to qualify to be restricted [s26AF(ea) refers].
Once a sponsor's L(A) application has been approved, and their clinical trial efficacy information is restricted, the TGA is not permitted to use the restricted information to evaluate another L(A) medicine application during the 5-year protection period, unless permission has been given by the sponsor.
The TGA will publish and maintain a list of L(A) medicines with data protection. This list will be updated when clinical trial efficacy information provided in an application for an L(A) medicine becomes restricted and can be viewed on the TGA website. Note that sponsors will be required to provide details of their journal article(s) once published.
A competitor will not be permitted to make a generic application of an innovator's medicine using the innovator's restricted information without written permission from the innovator.
Literature-based submissions are not eligible for data protection
Literature-based submissions for L(A) medicines are those that are based on publicly available information, such as a peer-reviewed journal article, a literature review or information that is part of general scientific knowledge. Therefore, the information provided in literature-based submissions for new intermediate indication-active ingredient(s) combination will not be eligible for data protection and will not be able to be classified as restricted information.
Furthermore, sponsors will need to certify that they are not relying on another sponsor's restricted information in their application unless they have received permission to do so (see The sponsor must own the information).
Sponsors must certify that they have not relied on restricted information (without permission) to support their L(A) application, regardless of whether it is a literature-based submission or not. Literature searches should identify and exclude any restricted information included in the TGA's list of L(A) medicines with data protection.
Therefore, any application for an L(A) medicine will lapse if it contains restricted information that is included in the TGA's list of L(A) medicines with data protection, as the sponsor will be considered to have made a false certification.
Clinical trial registries
Entering a clinical trial in a clinical trial registry helps to prevent selective publication and reporting of research outcomes, unnecessary duplication of research effort and is a means to keep patients and the public informed of upcoming or ongoing clinical trials into which they might want to enrol.
A clinical trial registry is the entity (either an organisation or website platform) that:
- houses and manages a formal list of clinical trials (the clinical trial register) being conducted (or that have recently been conducted) in Australia or internationally
- provides a mechanism for patients or others to register their interest in participating in a clinical trial
- generates a unique clinical trial registration number for each clinical trial in the clinical trial register.
Clinical trials that have been registered in a clinical trial registry listed in regulation 15AA of the Regulations are eligible for data protection. These include:
- The primary registries included in the World Health Organization's International Clinical Trials Registry Platform (ICTRP)[7], which currently includes 17 national and international clinical trial registries including the Australian New Zealand Clinical Trials Registry (ANZCTR).
- ClinicalTrials.gov[8].
A clinical trials register contains a minimum 24-item dataset about a clinical trial, which enables it to be easily and unequivocally identified. Information includes:
- the clinical trial identifier number provided by the primary clinical trial registry, other secondary identification numbers and date of registration
- study sponsor(s), collaborators and contact details
- ethics review details and an individual participant data (IPD) sharing statement, which indicates whether the sponsor will make clinical trial participant data available to researchers who were not part of the original study team
- study details, such as the title, interventions, inclusion/exclusion criteria, study type and size, primary and key secondary outcomes, summary results and completion date.
How to submit an L(A) medicine application and make a restricted information certification
Sponsors seeking to submit an L(A) application will need to submit the CTD Module 1.5 - Assessed listed medicines - Restricted information certification in CTD Module 1.5 of their application, which includes the following certifications:
- Restricted information has or has not been used to support the application
- permission details must be provided if the sponsor has used restricted information.
If the sponsor believes that their information is eligible to be restricted, the sponsor will need to certify that:
- The information is derived from a clinical trial in relation to an indication of the medicine.
- The clinical trial registry number(s) for the information are recorded in the World Health Organization's International Clinical Trials Registry Platform (ICTRP) or ClinicalTrials.gov.
- The information is not available in the public domain.
- The information relates to an intermediate indication.
- The new intermediate indication-active ingredient(s) combination is not included in the ARTG for another L(A) medicine.
- The indication is not included in the Therapeutic Goods (Permissible Indications) Determination.
- The sponsor owns the clinical trial efficacy information.
The certification also requires the sponsor to include the following information:
- Details of the new intermediate indication-active ingredient(s) combination that is supported by the clinical trial efficacy information.
- Clinical trial registry number(s) and universal trial number (if available).
- Details of the clinical trial efficacy information submitted in the dossier.
Successful L(A) medicine applications submitted via the 'New medicine' application type will be given a new AUST L(A) number; those submitted via the 'Variation to existing medicine'[9] application type may be able to maintain their existing AUST L(A) number. Guidance on completing the certifications can be found in Appendix 1.
Appendix 1
The information below will assist you in providing the required information for completing (and submitting) the CTD Module 1.5 - Assessed listed medicines - Restricted information certification (either for new ARTG entries or changes to existing ARTG entries made under s23 of the Act).
Completing the CTD Module 1.5 - Assessed listed medicines - Restricted information certification
Both Parts A and B of the certification must be submitted in CTD Module 1.5 of your submission.
Part A - Reliance on restricted information declaration
- Record the name of your medicine that is the subject of your L(A) application in the space provided.
- You will need to certify that you are not relying on restricted information (Section 1) or that the person (i.e. the sponsor) who is the owner of the restricted information [relating to another L(A) medicine] has given the Secretary of the TGA permission (Section 2) to rely on that information to support your application. Please refer to the TGA's list of L(A) medicines with data protection, which provides the current list of information that has been restricted).
- Certify in Section 1 that you have not relied on restricted information to support your application and complete Section 4 of the certification, if relevant. (If you are not submitting any human clinical trial information, e.g. L(A)1 clone, you do not need to complete Section 4); OR
- Certify in Section 2 that the person (i.e. sponsor), who is the owner of the restricted information [relating to another L(A) medicine], has given the Secretary of the TGA permission to rely on that information to support your application, and select one of the following options:
- Tick the box in Section 2(i) if you are the owner of the restricted information (i.e. the sponsor of the medicine to which the restricted information relates). You will also need to complete Section 3 and Section 4 (if relevant); OR
- Tick the box in Section 2(ii) if you are not the of the restricted information owner (i.e. not the sponsor of the medicine to which the restricted information relates), but have a letter from the owner giving the Secretary of the TGA permission to use that restricted information. You will also need to complete Section 3 and Section 4 (if relevant) and provide a letter of permission from the owner to use this restricted information in Module 1.5.
- In Section 3, provide information about the restricted information, including:
- the name of the medicine and AUST L(A) number associated with the restricted information
- the name of the sponsor of the medicine
- whether you have attached the letter of permission from the owner of the information to use this information for your application. If you are the owner of the information, i.e. the sponsor of the medicine to which the restricted information relates, you do not need to attach a letter of permission.
- the clinical trial registry number(s) (CTN) associated with the information
- the Universal Trial Number(s) (UTN, if available) associated with the information
- the data protection expiry date
- the intermediate indication-active ingredient(s) combination for which information is restricted.
- All sponsors providing human clinical trial studies to support their application will need to provide a list of all supporting information (whether restricted or not), including published and unpublished information in Section 4. You must:
- provide the clinical trial registry number(s) (CTN) associated with each clinical study
- provide the Universal Trial Number(s) (UTN) associated with each clinical study, if available
- include the title and author(s) of the document(s)
- provide the file name of the document(s) as they appear in the dossier
- if you are not submitting any human clinical trial information [e.g. L(A)1 clone], you are not required to complete Section 4.
- Ensure you enter your name (authorised officer's name[10]), your position (authorised officer's position), the name of the sponsor, signature and date at the bottom of Part A.
Part B - Eligibility for information to be restricted
- Record the name of your medicine which is the subject of your L(A) application in the space provided.
- You will need to select either 'yes' or 'no' to indicate whether you are requesting that information you have provided with this application be considered as restricted information.
- If you select 'no', you will only need to enter your name (authorised officer's name), your position (authorised officer's position), the name of the sponsor, signature and date at the end of Part B.
- If you select 'yes' you will need to:
- Enter each new intermediate indication-active ingredient(s) combination for which you are requesting to have information considered as restricted in Section 1. You will need to enter the new intermediate indication and the corresponding active ingredient(s).
- Provide a list of the information that you are requesting to be considered as restricted information in Section 2. In this list, you must:
- provide the clinical trial registry number(s) for each clinical study
- provide the Universal Trial Number(s) (if available) for each clinical study.
- include the title and author(s) of the document(s)
- provide the file name of the document(s) as they appear in the dossier
- Certify the following in the Certifications section at the end of Part B:
- the information is derived from a clinical trial that supports an indication of the L(A) medicine
- the trial number of the trial is specified in Part B Section 2 of this certification
- the clinical trial registry number(s) (CTN) and universal trial number (UTN) for the information are recorded in a prescribed clinical trial registry (i.e. WHO ICTRP or ClinicalTrials.gov)
- the information that you are requesting to be considered as restricted information is not available in the public domain (except for information contained within a prescribed clinical trial registry) and will not be made available to the public while the application is being evaluated
- the information is in relation to an intermediate indication
- the indication is not included in the Therapeutic Goods (Permissible Indications) Determination; i.e. the indication is not a permitted indication.
- the intermediate indication-active ingredient(s) combination is not included in the ARTG for another L(A) medicine
- you own the information i.e. you conducted or sponsored the study and have the legal right to use the clinical trial efficacy information.
- Ensure you enter your name (authorised officer's name[11]), your position (authorised officer's position), the name of the sponsor, signature and date at the bottom of Part B.
Accessing help
For L(A) medicine application and submission enquiries, please email nonprescriptionmedicines@health.gov.au with as much information as possible, such as a copy of the problem application or screenshots of any error messages received.
For questions about TBS-related issues and access you can contact the TBS helpdesk on ebs@health.gov.au.
Phone
You can phone Complementary medicines on 1800 020 653 or 02 6289 4627.
You can phone the TBS helpdesk on 1800 010 624.
Footnotes
- An intermediate-level indication is an indication that exceeds the criteria for low-level indications that appear on the list of permitted indications but is not a high-level indication; refer to Schedule 4, Item 8, paragraph (d) to the Regulations and the Indications section in the Assessed Listed Medicines Evidence Guidelines.
- Clinical trials refer to studies in which human participants volunteer to test one or more health-related interventions to evaluate the effects on health outcomes.
- Existing ingredients are those that have been evaluated by the TGA and are already listed in the Therapeutic Goods (Permissible Ingredients) Determination.
- Intermediate indications are defined in Paragraph 26AF(2)(c) of the Act and paragraph (d), Item 8, of Schedule 4 to the Regulations.
- As outlined in the Guideline for Good Clinical Practice, the purpose of the clinical trial study report is to document the results and interpretation of the trial. This should be prepared once the clinical trial has been completed or terminated. Guidance on how to compile the clinical study report is provided in the ICH Topic E3 Structure and Content of Clinical Study Reports (CPMP/ICH/137/95).
- The UTN, while not mandatory, provides a means to unambiguously identify a clinical trial by linking multiple records of the same trial.
- Primary registries in the WHO ICTRP meet specific criteria for content, quality and validity, accessibility, unique identification, technical capacity and administration. These registries meet the requirements of the International Committee of Medical Journal Editors (ICMJE). The ICMJE accepts publicly accessible registration in any registry in the ICTRP or ClinicalTrials.gov. The ICMJE endorses these registries as they meet several criteria (they must include a minimum 24-item trial registration dataset).
- ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. This does not meet the requirements of the ICMJE.
- This applies to products that exist in the ARTG that require changes to the listing, and where the variation is made under s23 of the Act and the provisions of the Therapeutic Goods (Groups) Order No. 1 of 2001 (‘the Groups Order’) apply.
- The authorised officer is the person authorised by the sponsor to sign this certification. This could be an employee or other officer of the sponsor, or an agent acting on behalf of the sponsor (e.g. a regulatory affairs consultant).
- The authorised officer is the person authorised by the sponsor to sign this certification. This could be an employee or other officer of the sponsor, or an agent acting on behalf of the sponsor (e.g. a regulatory affairs consultant).
Page history
Title changed from 'Data Protection Scheme for assessed listed medicines' to 'Explaining the Data Protection Scheme for assessed listed medicines' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Original publication
Title changed from 'Data Protection Scheme for assessed listed medicines' to 'Explaining the Data Protection Scheme for assessed listed medicines' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
Original publication

