What is the problem?
Ypsomed Australia is reminding users to ensure they use a compatible smart device and operating system (OS) when using the Mylife CamAPS FX app.
The product’s Instructions for Use (IFU) did not provide adequate clarity on smart device and OS compatibility.
When they are first released, the latest Android OS or Apple iOS update may not be compatible with the app and/or insulin pump. If the device or OS is updated while incompatible with the app, the app may stop working properly.
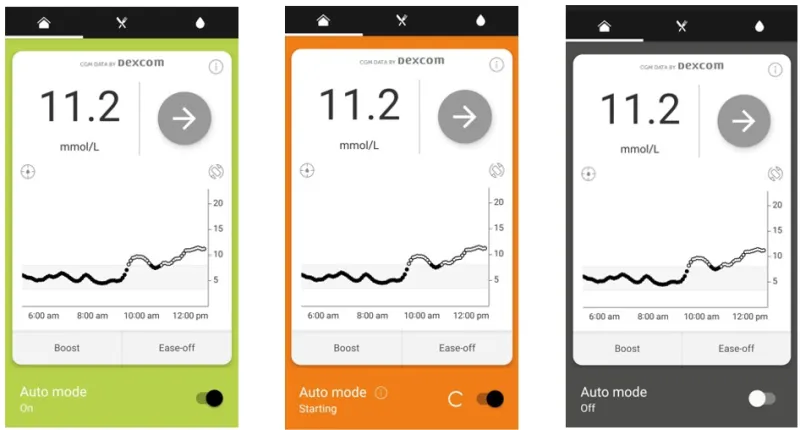
If this occurs, the app will revert to its safety default by delivering the pre-set pump basal rate. The colour of the home screen background will change from green (Auto mode ‘On’) to either orange (Auto mode ‘Attempting’) or grey (Auto mode ‘Off’).
| ARTG Number | App Versions |
|---|---|
| 489066 | Android 1.4(189) and iOS version 1.4(192), and all previous versions. |
What are the risks?
The problem could:
- Cause the app to stop working properly, changing the user’s insulin delivery to the safety default.
- Potentially cause hypoglycaemia (low blood glucose) or hyperglycaemia (high blood glucose).
What should I do?
- Review the updated IFU on smart device OS compatibility within the CamAPS FX app.
- Turn off auto-updates for your phones OS/iOS. Only update the operating system once the manufacturer’s website confirms compatibility with the new OS.
- If using an incompatible smart device or OS, your system may revert to manual mode (grey or orange screen) and deliver the preset basal rates. You can still administer bolus insulin doses from your app for your meals and see your sensor glucose data on the app.
Further information
Please contact Ypsomed Australia on 1800 447 042 for further information.
A software update has been released that will allow the app to be compatible with recent smart phone operating systems.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to our monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

