Consumers and health professionals are advised that Aspen Pharma and Arrow Pharma, in consultation with the TGA, are recalling one batch of acetazolamide 250 mg tablets (marketed in Australia as Diamox).
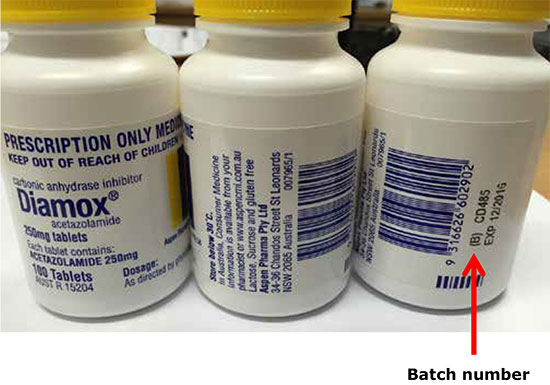
The affected batch number is CD485, with expiry date December 2016. No other batches of this product are affected.
Acetazolamide is a medicine used to treat oedema, epilepsy and glaucoma.
It has been identified that bottles of acetazolamide tablets from the affected batch may be contaminated with fungus - Penicillium and/or Aspergillus species.
Affected tablets may have a dusty, mouldy or abnormal appearance.
Information for consumers
If you or someone you care for is taking acetazolamide (with the brand name Diamox), check the batch number displayed on the bottle label under the barcode.
If the bottle is from batch number CD485, do not use it. Return it to the pharmacy where you purchased it for a refund or replacement.
If you have any questions or concerns about this issue, speak to your health professional or call Aspen Pharma on 1300 659 646 (select option 2).
Information for health professionals
Aspen Pharma and Arrow Pharma have written to all pharmacies and hospital pharmacies providing further information about this issue, including details of the recall procedure.
If you work in a hospital or pharmacy, check your stock for any bottles of acetazolamide 250 mg tablets from batch number CD485. Quarantine any bottles from the above batch and follow the recall procedure.
If you are treating patients who are taking acetazolamide, advise them of this issue.
If you have any questions or concerns about this issue, contact Aspen Pharma on 1300 659 646 (select option 2).
Reporting problems
Consumers and health professionals are encouraged to report problems with medicines or vaccines. Your report will contribute to the TGA's monitoring of these products.
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medicine or vaccine.

