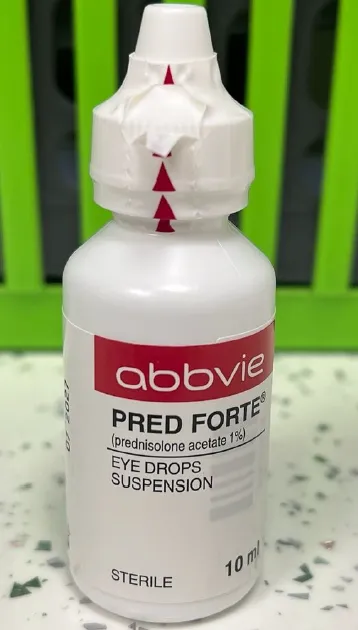
PRED FORTE prednisolone acetate 1% w/v eye drops, suspension (UK)
Section 19A approved medicine
PRED FORTE prednisolone acetate 1% w/v eye drops, suspension (UK)
Section 19A approval holder
Neon Healthcare Pty Ltd ABN 57 670 586 526
Phone
02 7255 8455
Approved until
Status
Current
Medicines in short supply/unavailable
PREDNEFRIN FORTE phenylephrine hydrochloride/prednisolone acetate eye drops bottle - ARTG 23235
Indication(s)
Severe inflammation (non-infectious) of the eye, such as acute iritis, iridocyclitis, scleritis, episcleritis, uveitis, resistant ocular allergy and inflammation following surgery (where no infectious aetiology is suspected), particularly where unusually rapid control of the inflammation is desired.
Images