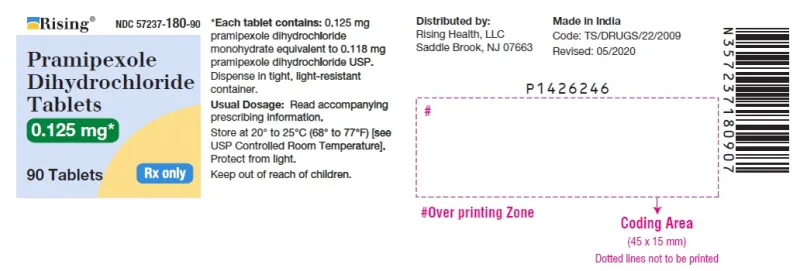
Pramipexole Dihydrochloride Tablets 0.125mg (Rising, USA)
Section 19A approved medicine
Pramipexole Dihydrochloride Tablets 0.125mg (Rising, USA)
Section 19A approval holder
Reach Pharmaceuticals Pty Ltd ABN 25 623 897 183
Phone
0422 429 648
Approved until
Status
Current
Medicines in short supply/unavailable
APO-PRAMIPEXOLE pramipexole dihydrochloride monohydrate 0.125 mg tablet blister pack - ARTG 227666
SIMPRAL pramipexole dihydrochloride monohydrate 0.125 mg tablet blister pack - ARTG 173139
SIMIPEX pramipexole dihydrochloride monohydrate 0.125 mg tablet blister pack - ARTG 172017
SIFROL pramipexole dihydrochloride monohydrate 125 microgram tablet blister pack - ARTG 67238
SIMPRAL pramipexole dihydrochloride monohydrate 0.125 mg tablet blister pack - ARTG 173139
SIMIPEX pramipexole dihydrochloride monohydrate 0.125 mg tablet blister pack - ARTG 172017
SIFROL pramipexole dihydrochloride monohydrate 125 microgram tablet blister pack - ARTG 67238
Indication(s)
The treatment of signs and symptoms of idiopathic Parkinson's disease. It may be used as monotherapy or in combination with levodopa.
The symptomatic treatment of primary Restless Legs Syndrome.
Images