We will have limited operations from 15:00 Wednesday 24 December 2025 (AEDT) until Friday 2 January 2026. Find out how to contact us during the holiday period.
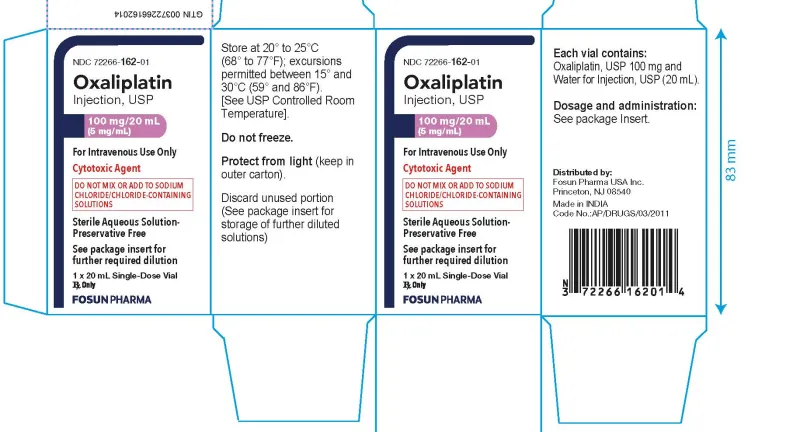
(Approval lapsed) Oxaliplatin Injection, USP 100 mg/20 mL (5 mg/mL) single-dose vial (Fosun Pharma, USA)
Section 19A approved medicine
(Approval lapsed) Oxaliplatin Injection, USP 100 mg/20 mL (5 mg/mL) single-dose vial (Fosun Pharma, USA)
Section 19A approval holder
Link Medical Products Pty Ltd ABN 73 010 971 516
Phone
1800 181 060
Approved until
Status
Expired
Medicines in short supply/unavailable
OXALIPLATIN ACCORD oxaliplatin 100 mg/20 mL concentrated injection vial - ARTG 352809
Indication(s)
Oxaliplatin in combination with fluorouracil and folinic acid is indicated for:
- Adjuvant treatment of stage III (Duke's C) colon cancer after complete resection of the primary tumour
- Treatment of advanced colorectal cancer
Images