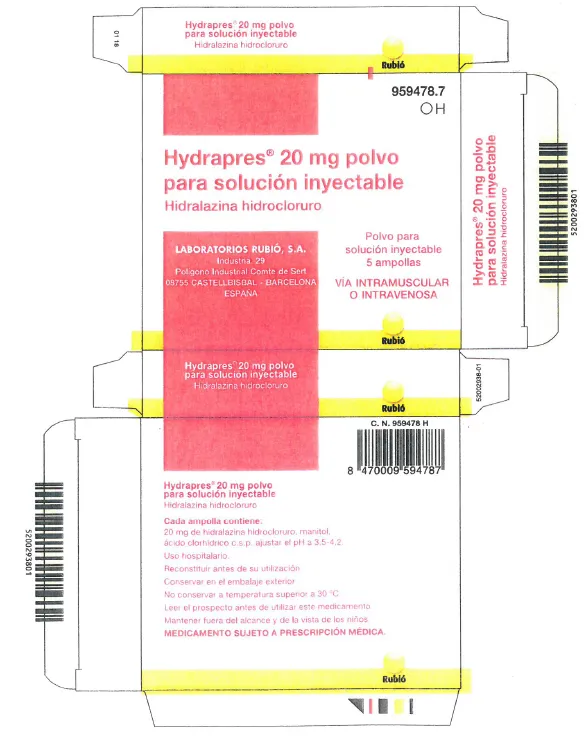
HYDRAPRES hydralazine hydrochloride 20mg powder for injectable solution ampoules (Spain)
Section 19A approved medicine
HYDRAPRES hydralazine hydrochloride 20mg powder for injectable solution ampoules (Spain)
Section 19A approval holder
Boucher & Muir Pty Ltd T/A Advanz Pharma ABN 58 000 140 474
Phone
1800 627 680
Approved until
Status
Current
Medicines in short supply/unavailable
APRESOLINE hydralazine hydrochloride 20mg powder for injection ampoule - ARTG 43290
Indication(s)
Hypertensive crises, especially during late pregnancy (pre-eclampsia and eclampsia)
Additional information
This approval commences on 01 August 2025.
Images