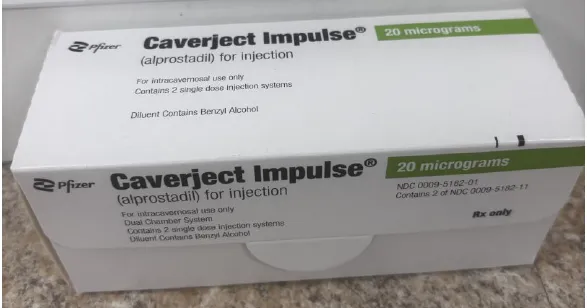
CAVERJECT IMPULSE alprostadil 20mcg for injection dual chamber system (USA)
Section 19A approved medicine
CAVERJECT IMPULSE alprostadil 20mcg for injection dual chamber system (USA)
Section 19A approval holder
Medsurge Healthcare Pty Ltd ABN 92 124 728 892
Phone
1300 788 261
Approved until
Status
Current
Medicines in short supply/unavailable
CAVERJECT IMPULSE alprostadil 20microgram powder for injection with 0.5mL diluent dual chamber cartridge ARTG - 90197
Indication(s)
Intracavernosal alprostadil (PGE1) is indicated for the treatment of erectile dysfunction in adult males. Intracavernosal alprostadil may be a useful adjunct to other diagnostic tests in the diagnosis of erectile dysfunction.
Images