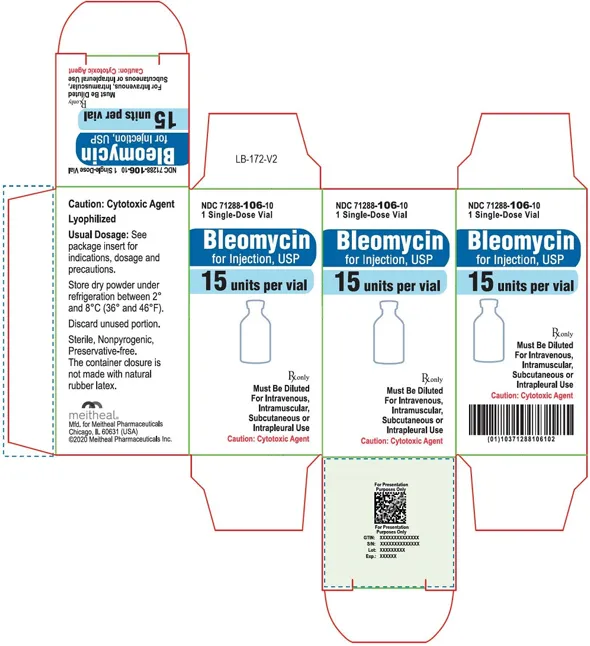
(Approval lapsed) Bleomycin for Injection, USP 15 units per vial (Meitheal, USA)
Section 19A approved medicine
(Approval lapsed) Bleomycin for Injection, USP 15 units per vial (Meitheal, USA)
Section 19A approval holder
ORSPEC Pharma Pty Ltd ABN 15 634 980 417
Phone
02 4339 4239
Approved until
Status
Expired
Medicines in short supply/unavailable
DBL BLEOMYCIN 15000IU (as sulfate) powder for injection vial - ARTG 42569
Indication(s)
Bleomycin is indicated for palliation and treatment adjuvant to surgery and radiation therapy of the following neoplasms:
- Squamous cell carcinoma of the skin, head and neck (primary indication)
- Squamous cell carcinoma of the larynx, penis and uterine cervix
- Choriocarcinoma and embryonal cell carcinoma of the testis
- Advanced Hodgkin’s disease and other lymphomas
Note: Use of bleomycin after radiation therapy is less successful than use before radiation therapy.
Bleomycin is bone marrow sparing and may be used when other cytotoxic agents are contraindicated.
Images