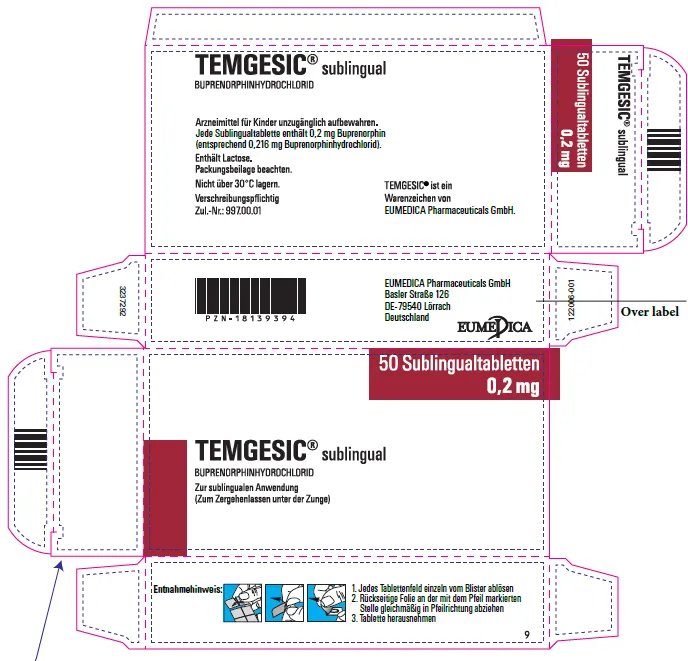
(Approval lapsed) TEMGESIC buprenorphine hydrochloride 0.2mg sublingual tablet (Germany)
Section 19A approved medicine
(Approval lapsed) TEMGESIC buprenorphine hydrochloride 0.2mg sublingual tablet (Germany)
Section 19A approval holder
Echo Therapeutics Pty Ltd ABN 92 628 298 699
Phone
1300 848 328
Approved until
Status
Expired
Medicines in short supply/unavailable
TEMGESIC buprenorphine 200 microgram (as hydrochloride) sublingual tablet blister pack - ARTG 34091
Indication(s)
TEMGESIC sublingual tablets are indicated for the short-term (not more than one week) management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
Images