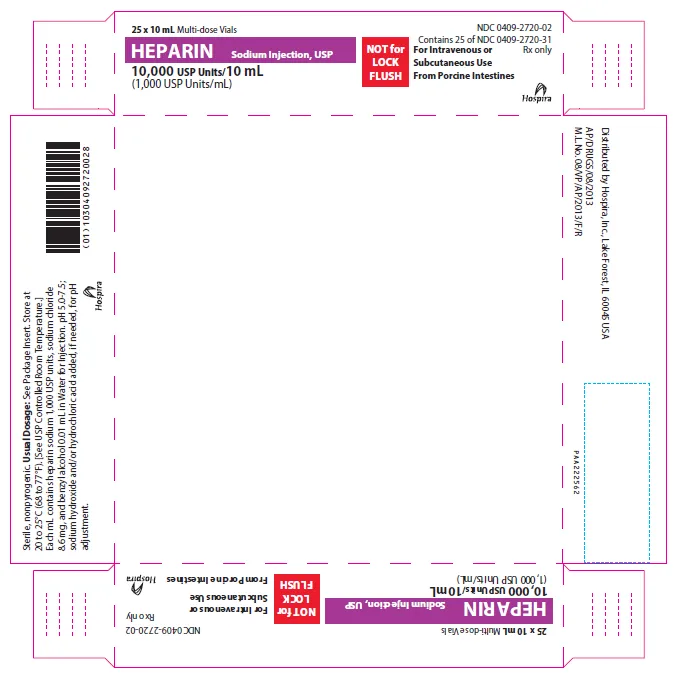
(Approval lapsed) Heparin sodium injection, USP 10,000 units/10 mL (1,000 units/mL) multi-dose vials for intravenous or subcutaneous use - CONTAINS PRESERVATIVE (Hospira, USA)
Section 19A approved medicine
(Approval lapsed) Heparin sodium injection, USP 10,000 units/10 mL (1,000 units/mL) multi-dose vials for intravenous or subcutaneous use - CONTAINS PRESERVATIVE (Hospira, USA)
Section 19A approval holder
Pfizer Australia Pty Ltd ABN 50 008 422 348
Phone
1800 675 229
Approved until
Status
Expired
Medicines in short supply/unavailable
HEPARIN SODIUM 5000IU/5mL (porcine mucous) injection ampoule - ARTG 49232
Indication(s)
Prophylaxis and treatment of pulmonary embolism and venous thrombosis. Prevention of thromboembolic complications arising as a result of cardiac and arterial surgery. As an anticoagulant during blood transfusions, extracorporeal circulation, and dialysis.
Images