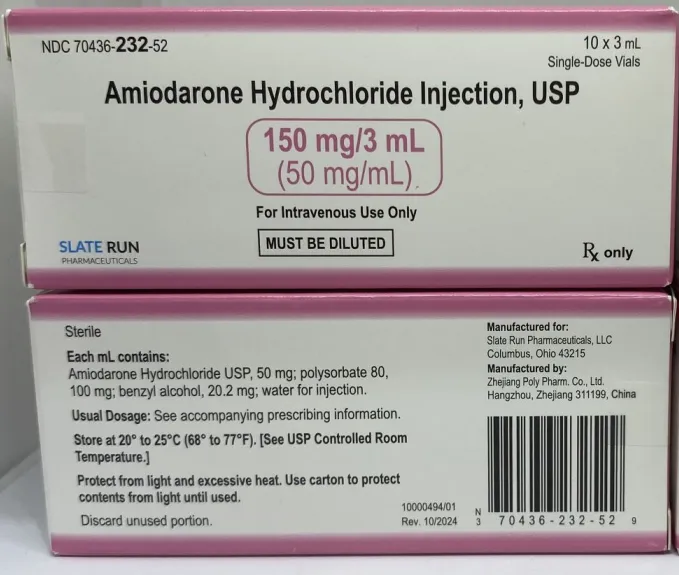
(Approval lapsed) Amiodarone hydrochloride injection, USP 150mg/3mL (50mg/mL) solution for injection single-dose vials (Slate Run, USA)
Severe cases of tachyarrhythmias (eg. Wolff-Parkinson-White Syndrome, supraventricular, nodal and ventricular tachycardias, atrial flutter and fibrillation, ventricular fibrillation) not responding to other therapy. Treatment should be initiated in hospital. It is recommended that the patient should be regularly monitored for possible toxicity (eg. thyroid function, chest X-ray, ophthalmological examination, liver function etc.) during the entire course of therapy and for several months after discontinuation. Amiodarone HHH injection may be used for treatment initiated in a hospital for severe cases of tachyarrhythmias (atrial, junctional and ventricular) not responding to other therapy and when a rapid response is required. Amiodarone HHH injection should only be used where facilities exist for cardiac monitoring and defibrillation should the need arise.