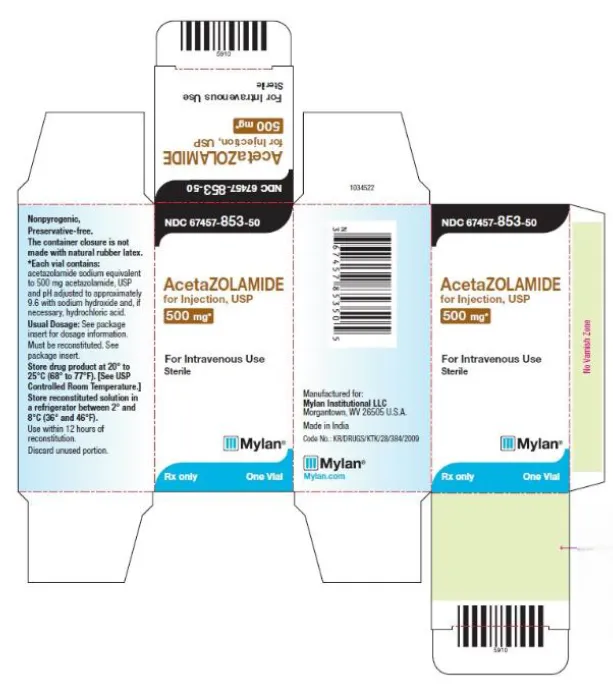
Acetazolamide for injection, USP 500 mg (Mylan, USA)
Section 19A approved medicine
Acetazolamide for injection, USP 500 mg (Mylan, USA)
Section 19A approval holder
Phero Pharma Pty Ltd ABN 96 673 730 231
Phone
02 94209199
Approved until
Status
Current
Medicines in short supply/unavailable
Glaumox Acetazolamide sodium 500 mg - ARTG 142075
Indication(s)
For adjunctive treatment of: oedema due to congestive heart failure; drug-induced oedema; centrencephalic epilepsies (petit mal, unlocalised seizures); chronic simple (open-angle) glaucoma, secondary glaucoma and preoperatively in acute angle-closure glaucoma where delay of surgery is desired in order to lower intraocular pressure.
Images