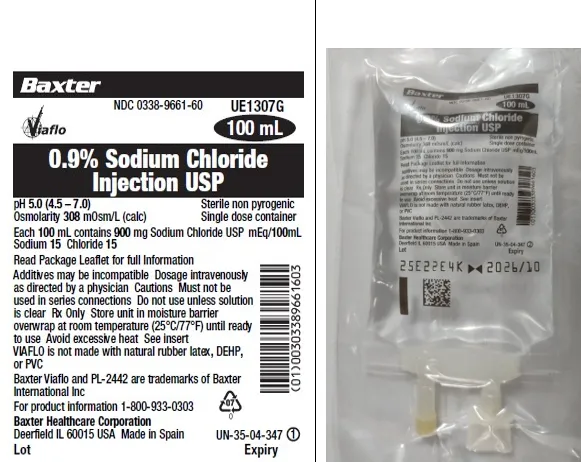
0.9% Sodium Chloride Injection USP in VIAFLO Plastic Container 100 mL (Baxter USA)
Section 19A approved medicine
0.9% Sodium Chloride Injection USP in VIAFLO Plastic Container 100 mL (Baxter USA)
Section 19A approval holder
Baxter Healthcare Pty Ltd ABN 43 000 392 781
Phone
1800 229 837
Approved until
Status
Current
Medicines in short supply/unavailable
BAXTER 0.9% SODIUM CHLORIDE 900mg/100mL injection BP bag - ARTG 48515
Indication(s)
Indicated for extracellular fluid replacement and for restoring or maintaining the concentration of sodium and chloride ions.
Images