Therapeutic goods advertising requirements apply to medical devices, equipment, and products.
Advertisements promote a therapeutic good through a statement, image, or design.
Medical devices you can advertise
Most medical devices can be advertised to the public as long as the advertising complies with the Advertising Code. For more information, see Applying the Advertising Code.
You can advertise your personalised medical device to consumers if it is:
- included in the Australian Register of Therapeutic Goods (ARTG) (see Medical device inclusion process) or
- exempt from ARTG inclusion because it is a patient-matched medical device (PMMD) or
- exempt from ARTG inclusion because it meets our definition of a custom-made medical device (CMMD).
Note
Even if your device does not meet our legislated definition of a CMMD, you can still use the term ‘custom-made’ when advertising other personalised devices. This is because ‘custom-made’ has a recognised meaning in everyday language.
What you can’t advertise
Medical devices containing substances listed in Schedule 3, 4 or 8 of the Poisons Standard (except those in Appendix H) can't be advertised.
Things you can’t say in advertising
References to serious conditions, diseases, ailments and defects are restricted and need TGA approval before use. Using words like "prevent, cure, treat, or diagnose serious conditions" is prohibited.
See Restricted and prohibited representations in advertising for more information.
We also have rules about who can make testimonials and endorsements. See Applying the Advertising Code rules: testimonials and endorsements.
Advertising to health professionals
You can provide more information in advertising aimed at health professionals. This only applies if you take steps to ensure consumers can’t access this material. See Advertising to health professionals so that consumer rules do not apply.
Examples
Compliant

- Advertisements must provide balanced, factual, and substantiated information.
- Advertisements must contain and prominently display either of the following mandatory statements:
- Always follow the directions for use
- Always read the label and follow the directions for use
- Consumers must be able to locate or request details about clinical studies mentioned.
- Scientific or clinical research references must identify the financial sponsor (if known).
A digital advertisement for BeanzAligners.
At the top, the BeanzAligners logo is displayed with the word “Sponsored.” Below, text reads:
“Shine through your smile with removable aligners:
• This treatment is a series of orthodontic aligners that gently move your teeth over time.
• Studies show our aligners will straighten your smile.
• ALWAYS FOLLOW THE DIRECTIONS FOR USE.”
Further down, a large heading says:
“Studies show our aligners will straighten your smile.”
Underneath, smaller text reads:
“Ask your dentist if aligners are right for you.”
The main image features a woman smiling.
At the bottom, there 3 fictional references to studies are listed. This is followed by the statement: “Studies funded by BeanzAligners.”
A website link and a button labelled “Learn More” appear at the bottom.
Example
Non-compliant
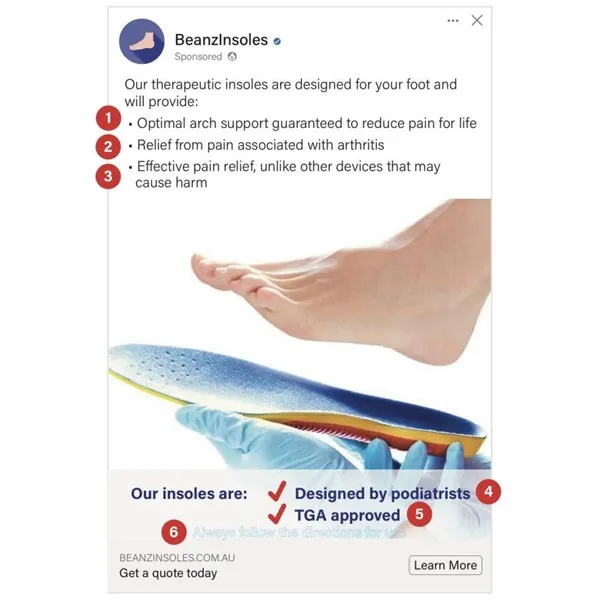
This advertisement is non-compliant because:
- It states or implies that the advertised good is effective in all cases, or a guaranteed cure.
- It refers to a serious conditions and disease. You can only include this with TGA approval.
- It states or implies that another device is ineffective or harmful
- Health professionals or medical researchers can not endorse products in advertisements.
- It includes the phrase ’TGA approved’.
- Does not contain the mandatory statements:
- Always follow the directions for use.
- Always read the label and follow the directions for use.
This image is an advertisement for insoles. The ad is presented as a sponsored social media post.
The top of the image shows the company logo and name "BeanzInsoles". The main text states "Our therapeutic insoles are designed for your foot and will provide:
- "Optimal arch support guaranteed to reduce pain for life"
- "Relief from pain associated with arthritis"
- "Effective pain relief, unlike other devices that may cause harm"
The central part of the image shows a bare foot hovering above a blue and yellow insole. Below the image, text reads "Designed by podiatrists" and "TGA approved".
At the bottom, there's a website URL and a "Learn More" button.
Using comparisons
You must not advertise a medical device in a way that suggests other devices are harmful or ineffectual. This includes any statements, images, or designs that make such comparisons, whether directly or indirectly.
For example, aligners and braces are both used to treat misaligned teeth. Comparisons can be made between aligners and braces in advertising, but the comparisons cannot express or imply that the other medical device is harmful or ineffectual.
Examples of statements that could be used when comparing aligners and braces:
Is your child resisting traditional braces? They may prefer using aligners…
On vacation? Forgotten your aligners? Should’ve gone with braces!
Aligners achieve faster results than traditional braces (when used as directed by your dentist).
This claim includes a qualifier in brackets and avoids a blanket statement. Blanket statements can mislead consumers.
This comparison could be used if the advertisement includes references to robust scientific studies that support this claim.
Braces can correct more extensive misalignments in your smile compared to aligners (by exerting more physical force than aligners).
This comparison could be used if the advertisement includes references to robust scientific studies that support this claim.
Unlike braces, aligners allow you to enjoy your favourite crunchy foods!
This statement is factual and does not express or imply that the other medical device is harmful or ineffectual.
90% of customers say they prefer aligners over braces.
This comparison could be used if the advertisement includes references to robust scientific studies that support this claim.
Examples of statements that should not be used when comparing aligners and braces:
Braces are ineffective at straightening teeth compared to aligners.
Comparisons cannot express or imply that other medical devices are ineffective.
Aligners are better than braces.
This statement is unsubstantiated and could be considered misleading for implying that braces are ineffective compared to aligners.
Braces take forever to straighten teeth compared to aligners.
This statement could be considered to be misleading and exaggerate how long it takes braces to straighten teeth, implying that braces are ineffectual.
Related links
Page history
Removed outdated information related to transition notifications.
Added details about testimonials and endorsements, advertising to health professionals, and using comparisons.
Republished the compliant example.
Removed compliant example.
Original publication.
Removed outdated information related to transition notifications.
Added details about testimonials and endorsements, advertising to health professionals, and using comparisons.
Republished the compliant example.
Removed compliant example.
Original publication.

