Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is to assist manufacturers of active medical devices, including software-based medical devices, in correctly classifying their devices.
This guidance does not apply to IVD medical devices. See classification of IVD medical devices for information about IVD medical devices.
Legislation
Introduction
Medical devices are classified according to the level of harm they may pose to users or patients. There is a four-tier classification system for medical devices:
- Class I (lowest classification)
- Class IIa
- Class IIb
- Class III (highest classification).
The higher classification level, the higher the level of regulatory oversight.
Classification is used to determine the minimum conformity assessment (CA) procedures (or comparable overseas regulator evidence requirements) to be determined by the manufacturer prior to an application being made for inclusion of the device in the Australian Register of Therapeutic Goods (ARTG).
An active medical device is defined as a medical device intended by its manufacturer:
- to depend on a source of electrical energy or other source of energy (other than a source of energy generated directly by a human being or gravity) for its operation; and
- to act by converting this energy; but
- is not a medical device intended by the manufacturer to transmit energy, a substance, or any other element, between an active medical device and a human being without any significant change in the energy, substance or other element being transmitted.
Software-based medical devices are active medical devices. This includes software that is a medical device itself and medical devices that incorporate software.
The classification process
Medical devices are classified according to the medical device classification rules in Schedule 2 (Part 4) of the Therapeutic Goods (Medical Devices) Regulations 2002 (the Regulations).
The principles for applying the classification rules are provided under Part 3, Division 3.1, Regulation 3.3 - Principles for applying the classification rules.
The particular principles to be considered are:
- if a medical device is designed to be used in combination with another medical device, each of the devices is classified separately
- an accessory to a medical device is classified separately from the medical device
- if a medical device is driven, or influenced, by an item of software, the software has the same classification as the medical device.
The manufacturer is responsible for determining the classification of the device. The regulatory requirements applied to the manufacturer correlate with classification level.
This guidance should be read together with the Regulations
This document is intended to be an aid to understanding how to classify your active medical device, including software-based medical devices. Other classification rules not detailed in this document may also apply.
Applying the classification rules
The classification rules are applied according to the manufacturer’s intended purpose, taking into account how the device works.
Manufacturers must consider all the classification rules in classifying their medical device. Where more than one rule applies, the device must be classified at the highest applicable level.
It is likely that more than one rule will apply, especially to complex and multi-functional medical devices. Each individual function must be considered against the classification rules. The highest possible classification will apply to the device as a whole for the purpose of its inclusion in the ARTG.
Devices for export only are always classified as Class I.
Review classification if functionality of the device changes
Manufacturers of medical devices must review original classifications periodically to check they are still valid.
The classification of device may change if:
- the intended purpose or indications change over time
- functionality is added or removed
- the manufacturer makes different claims about the device.
Rules to consider depending on the intended purpose of your device
The following table summarises which rules apply to active medical devices intended for
particular purposes.
| Intended to be used for… | Rules that may apply |
|---|---|
| General (default) | Rule 4.1 |
| Recording patient images | Rule 4.1, 5.4(1) |
| Anatomical models | Rule 4.1, 4.3, 5.4(2), 5.4(3) |
| Diagnosis | Rule 4.1, 4.3, 4.5, 5.4, 5.7 |
| Screening | Rule 4.1, 4.3, 4.5 |
| Monitoring | Rule 4.1, 4.3, 4.6, 5.4, 5.7 |
| Investigation of the anatomy or physiology | Rule 4.1, 5.4 |
| Specifying or recommending a treatment or intervention | Rule 4.1, 4.7 |
| Therapy | Rule 4.1, 4.2, 4.4, 4.8, 5.7 |
The various classification rules are discussed in more detail below.
Classification rules
There are several classification rules for active medical devices that may apply depending on the intended purpose of the device. The rules have been grouped according to the following:
- detecting, diagnosing, screening, monitoring, investigation
- therapy
- recording patient images and anatomical models
- medical devices intended for contraception or prevention of sexually transmitted diseases
- general rule for any other active medical device.
Detecting, diagnosing, screening, monitoring, investigation
Rule 4.3 - Active medical devices for diagnosis (includes monitoring)
This rule is specific to active medical devices for diagnosis.
For the application of this rule, an active medical device for diagnosis means an active medical device intended by the manufacturer to be used on a human being, either alone or in combination with another medical device, to supply information for the purpose of detecting, diagnosing, monitoring or treating physiological conditions, states of health, illnesses or congenital deformities.
This rule applies to active medical devices for diagnosis intended to:
- supply energy that will be absorbed by a patient’s body (except where the device is only intended to illuminate the patient’s body in the visible spectrum)
- image the in vivo distribution of radiopharmaceuticals in a patient
- allow the direct diagnosis or monitoring of vital physiological processes in a patient.
Active medical devices for diagnosis (includes monitoring)
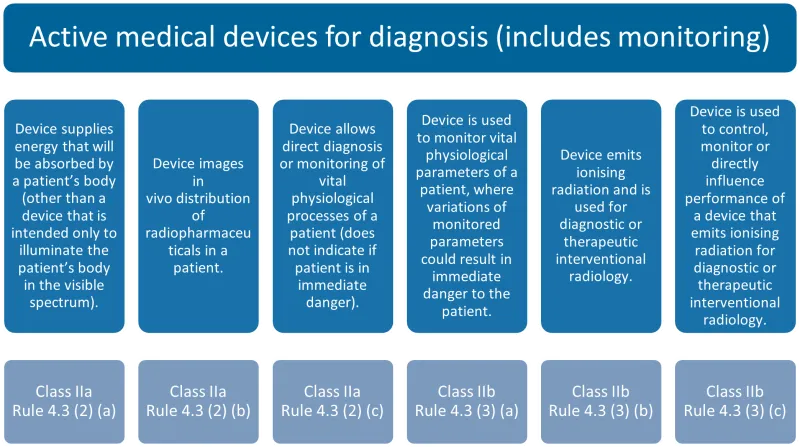
This chart categorises active medical devices for diagnosis and monitoring into 6 types, each with its corresponding classification and rule.
The categories are:
- Devices supplying energy absorbed by a patient's body (excluding visible light illumination): Class IIa, Rule 4.3 (2) (a)
- Devices imaging in vivo distribution of radiopharmaceuticals: Class IIa, Rule 4.3 (2) (b)
- Devices for direct diagnosis or monitoring of vital physiological processes (non-immediate danger): Class IIa, Rule 4.3 (2) (c)
- Devices monitoring vital physiological parameters where variations could result in immediate danger: Class IIb, Rule 4.3 (3) (a)
- Devices emitting ionising radiation for diagnostic or therapeutic interventional radiology: Class IIb, Rule 4.3 (3) (b)
- Devices controlling, monitoring, or directly influencing the performance of devices emitting ionising radiation for diagnostic or therapeutic interventional radiology: Class IIb, Rule 4.3 (3) (c).
Direct diagnosis or monitoring of vital physiological processes
For the purpose of this rule, vital physiological process means a process necessary to sustain life, the indicators of which may include any one or more of the following:
- respiration
- heart rate
- cerebral function
- blood gases
- blood pressure
- body temperature.
Where these parameters are monitored for the purpose of detecting, diagnosing, monitoring or treating physiological conditions, states of illness or congenital deformities.
This rule also incorporates the significance of the parameters being monitored and whether they could signify or identify immediate danger to the patient.
For example, medical devices intended to be used for continuous surveillance of vital physiological processes in anaesthesia, intensive care or emergency care are Class IIb, while medical devices intended to be used to obtain readings of vital physiological signals in routine check-ups and in self-monitoring are Class IIa.
Note that products intended to be used by consumers for monitoring heart rate or rhythm solely for general wellness or fitness purposes are not medical devices - see Therapeutic Goods (Excluded Goods) Determination 2018.
| Examples | Rule and classification |
|---|---|
| Magnetic resonance equipment; pulp testers; evoked response stimulators; general purpose diagnostic ultrasound. | 4.3(2)(a) A device to supply energy that will be absorbed by a patient’s body (except a device that illuminates the patient’s body in the visible spectrum) Class IIa. |
| Gamma cameras; positron emission tomography; single photon emission computer tomography. | 4.3(2)(b) A device to be used to image in vivo distribution of radiopharmaceuticals in patients Class IIa. |
| Electrocardiographs; electroencephalographs; cardioscopes with or without pacing pulse indicators; electronic thermometers; electronic blood pressure measuring equipment. | 4.3(2)(c) A device used for direct diagnosis or monitoring of vital physiological processes of a patient, excluding devices mentioned in the previous entry Class IIa. |
| Intensive-care monitoring systems; blood gas analysers used in open-heart surgery; cardioscopes; apnoea monitor that monitors the respiratory rate and alerts the carer when a life-threatening episode occurs. | 4.3(3)(a) A device to monitor vital physiological parameters of a patient, and the nature of variations monitored could result in immediate danger to the patient Class IIb. |
| Diagnostic X-ray sources; linear accelerators. | 4.3(3)(b) A device to emit ionising radiation and to be used for diagnostic or therapeutic interventional radiology Class IIb. |
| Auto-exposure control systems; radiotherapy after-loading control systems. | 4.3(3)(c) A device to control, monitor or directly influence the performance of a device in the previous entry Class IIb. |
Rule 4.5 - Diagnosis or screening for a disease or condition
Rule 4.5 applies to active medical devices intended to be used to process data or information in order to:
- provide a diagnosis of a disease or condition. or
- screen for the potential presence of a disease or condition.
Screening is the detection of potential disease indicators in otherwise healthy, asymptomatic but at-risk individuals, in order to determine whether a confirmatory diagnostic test is warranted.
A higher classification applies if the device performs all the decision-making itself and provides a diagnosis or a screening result to the user, who may be either a layperson or a health professional.
A lower classification may apply if the device only provides information to a relevant health professional to assist them in diagnosing or screening for a disease or condition, and the health professional is responsible for the final diagnostic decision-making.
The classification of a device also depends on the seriousness of the disease or condition diagnosed or screened for and whether there would be any associated public health risk.
Note: this rule does not apply to a device that provides a diagnosis or screening result, either to itself or to another medical device.
Diagnosis or screening for a disease or condition
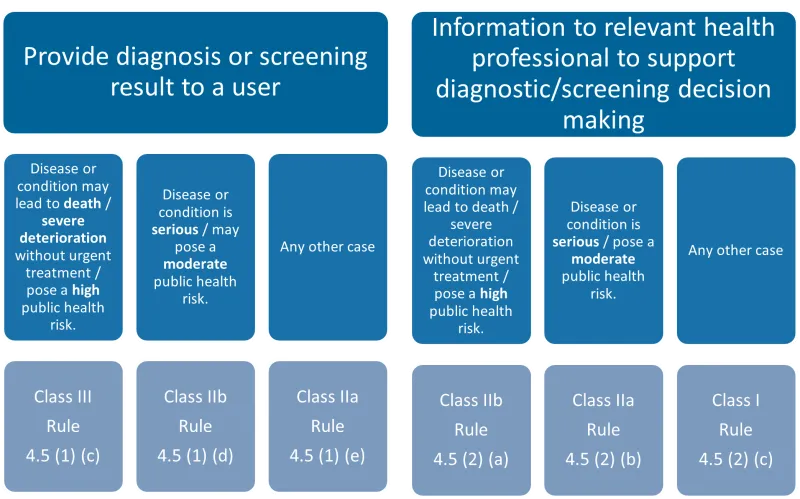
This chart categorises medical software into two main functions, each with three sub-categories based on the severity of the disease or condition. The categories are:
- Provide diagnosis or screening result to a user:
- Disease or condition may lead to death/severe deterioration without urgent treatment/pose a high public health risk: Class III, Rule 4.5 (1) (c)
- Disease or condition is serious/may pose a moderate public health risk: Class IIb, Rule 4.5 (1) (d)
- Any other case: Class IIa, Rule 4.5 (1) (e)
- Information to relevant health professional to support diagnostic/screening decision making:
- Disease or condition may lead to death/severe deterioration without urgent treatment/pose a high public health risk: Class IIb, Rule 4.5 (2) (a)
- Disease or condition is serious/pose a moderate public health risk: Class IIa, Rule 4.5 (2) (b)
- Any other case: Class I, Rule 4.5 (2) (c).
Relevant health professional
For the purposes of classification, a relevant health professional is one with the appropriate expertise to use the information from the device to assist them in making a decision. For example, an oncologist would be a relevant health professional for diagnosis and treatment recommendations for certain forms of cancer.
The classification of an active medical device is one class lower where it does not make the diagnosis or screening result itself, and instead provides information to a relevant health professional to assist them in diagnosing or screening for a disease or condition.
Seriousness of disease or condition
'Serious' has the meaning defined in the Regulations.
Serious means a condition, ailment or defect that is:
- generally accepted as not being appropriate to be diagnosed or treated without consulting a medical practitioner, dentist or other kind of health care worker registered under a law of a state or territory; or
- generally accepted to be beyond the ability of the average person to evaluate accurately, or treat safely, without supervision by a medical practitioner, dentist or other kind of health care worker registered under a law of a state or territory.
Serious disease means a disease that:
- may result in death or long-term disability; and
- may be incurable or require major therapeutic interventions; and
- must be diagnosed accurately, to mitigate the public health impact of the disease
Urgent treatment
Urgent treatment refers to critical situations or conditions where timely diagnosis or treatment is necessary to avoid death or serious deterioration in the person’s health. Such critical situations or conditions would include:
- life threatening, including incurable, states of health
- diseases or conditions that require major therapeutic intervention
- situations in which timely intervention could prevent the rapid progression of a serious disease or condition, such as in cases of malignant melanoma.
| Examples where the diagnostic or screening decision is performed by device and provided to the user | Rule and classification |
|---|---|
| A consumer mobile phone app intended to analyse an image of a mole to screen for malignant melanoma. | 4.5(1)(c)(i) In the case of a disease or condition that may lead to the death of a person, or a severe deterioration in the state of a person’s health, without urgent treatment Class III. |
A device intended to diagnose emphysema from computed tomography (CT) scans. A device indented to screen for abnormalities in heart valves by analysing a transoesophageal echocardiogram (TTE). | 4.5(1)(d) In the case of a serious disease or serious condition Class IIb. |
| Examples where information is provided to a relevant health professional to make a diagnosis of or screen for a disease or condition | Rule and classification |
|---|---|
An app intended to analyse a mammogram image and provides information to a health professional to aid in diagnosing breast cancer. *Note, this rule would not apply to a medical device that only records a medical image. | 4.5(2)(a)(i) In the case of a disease or condition that may lead to the death of a person, or a severe deterioration in the state of a person’s health, without urgent treatment Class IIb. |
| A computer program intended to provide information to a general practitioner (GP) for the purposes of aiding the GP to make a diagnosis of diabetes. | 4.5(2)(b) In the case of a serious disease or serious condition Class IIa. |
Rule 4.6 - monitoring the state or progression of a disease or condition
Rule 4.6 applies to active medical devices intended to provide information used to monitor the state or progression of a disease or condition of a person. This also includes monitoring of parameters in relation to the state or progression of a disease or condition of a person.
Medical devices covered under this rule process data in order to provide an output in the form of information, or parameters, that indicate the state of a disease or condition of a person. The data used as an input to such a device may include multiple or single sources and could consist of, for example, data provided by physiologic sensors such as heart rate monitors, data from other medical devices such as those used in an intensive care unit.
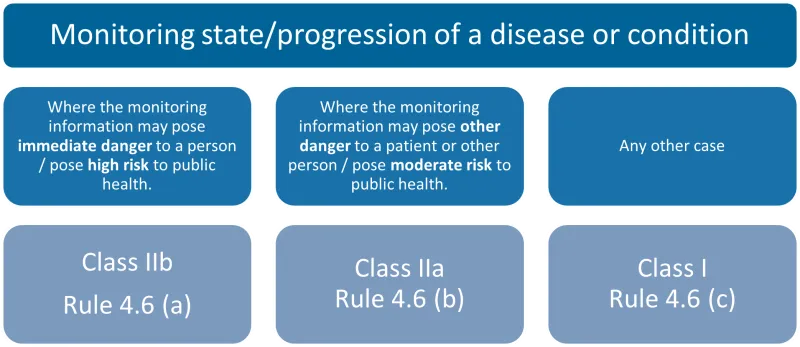
This chart categorises devices for monitoring the state/progression of a disease or condition into three types, each with its corresponding classification and rule.
The categories are:
- where the monitoring information may pose immediate danger to a person/pose high risk to public health: Class IIb, Rule 4.6 (a)
- where the monitoring information may pose other danger to a patient or other person/pose moderate risk to public health: Class IIa, Rule 4.6 (b)
- any other case: Class I, Rule 4.6 (c).
What ‘monitoring’ means
For the purposes of this rule ‘monitoring’ refers to the monitoring of a condition, disease, injury or disability. It also includes monitoring of physiological parameters of a person to monitor, for example, kidney function or muscle tone.
This does not include indirect monitoring activities, such as regularly reviewing an individual’s health records to determine if they meet the criteria to participate in a screening program for a particular disease or are due for a particular medical assessment.
Note that products intended to be used by consumers for monitoring heart rate or rhythm solely for general wellness or fitness purposes are not medical devices.
| Examples | Rule and classification |
|---|---|
| Software to analyse imagery from a gamma camera, recorded during a SPECT scan, to track and monitor the progression of heart disease from cardiac muscle blood-flow. | 4.6(a) In the case where the information to be provided could indicate that the person or another person may be in immediate danger or that there may be a high risk to public health. Class IIb. |
An app, which receives data via Bluetooth from an electromyography device, to monitor muscle fibre response in a person with muscular dystrophy.
| 4.6(b) In the case where the information to be provided could indicate that the person or another person may be in other danger or that there may be a moderate risk to public health. Class IIa. |
| A handheld auto-refractor to allow in-home monitoring of the progression of presbyopia (age-related long-sightedness). A cloud-based deep learning neural network to monitor patient recovery from shingles (herpes zoster) from uploaded images of shingles rash. | 4.6(c) In any other case Class I. |
Rule 4.7 - Specifying and recommending treatment or intervention
This rule applies to active medical devices intended to be used to process data or information in order to specify or recommend a treatment or intervention.
The classification depends on the seriousness of the risk of harm to a person and any associated public health risk.
A higher classification applies if all decision-making is performed by the device itself, and a treatment or intervention is specified directly to the user, who may be either a layperson or a health professional. A higher classification also applies if a treatment recommendation is provided to a layperson (rather than a recommendation to a health professional).
A lower classification applies if a relevant health professional is responsible for final decision-making, with the device providing information to the relevant health professional to aid or enable them to make a treatment or intervention decision.
The term relevant health professional is intended to emphasise the role of the health professional in being the decision-maker.
Note: this rule does not apply to a device that specifies or recommends a treatment or an intervention to itself or to another medical device.
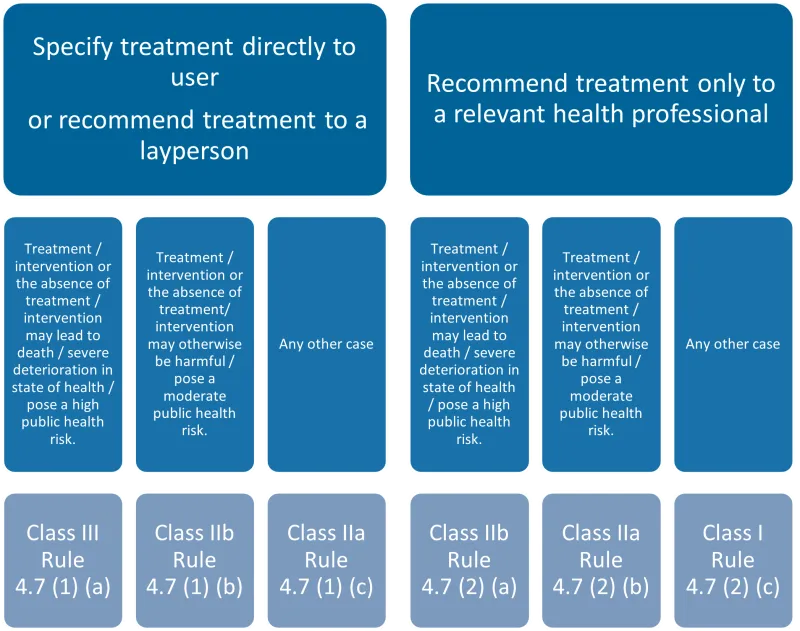
This chart categorises medical devices that specify or recommend treatment into two main groups, each with three subcategories based on the potential risk level.
The categories are:
- Specify treatment directly to user or recommend treatment to a layperson:
- Treatment/intervention or its absence may lead to death/severe deterioration in health/pose a high public health risk: Class III, Rule 4.7 (1) (a).
- Treatment/intervention or its absence may otherwise be harmful/pose a moderate public health risk: Class IIb, Rule 4.7 (1) (b).
- Any other case: Class IIa, Rule 4.7 (1) (c).
- Recommend treatment only to a relevant health professional:
- Treatment/intervention or its absence may lead to death/severe deterioration in health/pose a high public health risk: Class IIb, Rule 4.7 (2) (a).
- Treatment/intervention or its absence may otherwise be harmful/pose a moderate public health risk: Class IIa, Rule 4.7 (2) (b).
- Any other case: Class I, Rule 4.7 (2) (c).
Relevant health professional
The term relevant describes a health professional with appropriate expertise who uses the provided information to assist them in making a decision about the treatment. For example, an orthopaedic surgeon would be a relevant health professional for performing a hip replacement.
This rule covers devices intended to:
- Specify a treatment or intervention to a user (where the software makes the decision on the appropriate specific treatment or intervention) regardless of whether this is a layperson or healthcare professional. This will result in a higher classification.
- Provide a recommendation to a layperson about treatment or intervention. These devices also have a higher classification than those intended to provide a recommendation to a relevant healthcare professional.
- Provide a recommendation to a relevant healthcare professional in which case a lower classification may apply because the device is not intended to replace the clinical judgement of the health professional.
Criticality of the treatment/intervention being specified or recommended
The rule also takes into account situations where the treatment, or absence of the treatment, may result in the imminent death of a person or severe deterioration of their health.
Consideration must be given to whether a particular treatment is time-critical, as well as the risks relating to the treatment or intervention itself, for example, surgery versus minor therapeutic interventions.
| Examples where information is provided directly to the user | Rule and classification |
|---|---|
| An active medical device intended to specify particular surgical parameters for robotic assisted cardiac bypass surgery. The parameters are specified to a cardiac surgeon, who uses these to configure the surgical robot. | 4.7(1)(a)(i) Where the absence of the treatment or intervention or where the treatment or intervention itself may lead to the death of a person or a severe deterioration in the state of a person’s health Class III. |
| Example where information is provided to relevant health professional | Rule & Classification |
|---|---|
| A software application intended to recommend options for coronary artery bypass grafting surgery to a cardiac surgeon. | 4.7(2)(a)(i) Where the absence of the treatment or intervention or where the treatment or intervention itself may lead to the death of a person or a severe deterioration in the state of a person’s health Class IIb. |
| Software intended to recommend corneal surgical/transplantation options to an eye surgeon for the purpose of the eye surgeon treating keratoconus in a patient. | 4.7(2)(b)(i) Where the absence of the treatment or intervention or where the treatment or intervention itself may otherwise be harmful to a person Class IIa. |
Therapy
Rule 4.2 - Active medical devices for therapy
Administering and extracting energy
This rule is specific to active medical devices for therapy. For the application of this rule, an active medical device for therapy is one that is intended by the manufacturer to be used on a human being, either alone or in combination with another medical device, to support, modify, replace or restore biological functions or structures for the purpose of treating or alleviating an illness, injury or disability.
Active medical devices for therapy are intended to be used to administer energy to a patient, or exchange energy to or from a patient.
From 25 November 2021, rule 4.2 will be amended to classify active medical devices for therapy that include a diagnostic function the purpose of which is to significantly determine patient management by the device as Class III. For more information, see Reclassification of active medical devices for therapy with a diagnostic function.

This chart categorises active devices for therapy into three types, each with its corresponding classification and rule.
The categories are:
- Devices used to administer or exchange energy to/from a patient, where the energy is non-hazardous: Class IIa, Rule 4.2 (1)
- Devices used to administer or exchange energy to/from a patient in a potentially hazardous way: Class IIb, Rule 4.2 (2)
- Devices used to control, monitor, or directly influence a device that administers or exchanges energy to/from a patient in a potentially hazardous way: Class IIb, Rule 4.2 (3).
Active implantable medical devices
Note that Rule 5.7 applies if an active device for therapy:
- is implantable; or
- is an implantable accessory to an active implantable device; or
- controls, monitors, or directly influences the performance of an active implantable medical device.
| Examples | Rule and classification |
|---|---|
Electrical - magnetic and electromagnetic energy muscle stimulators; external bone growth stimulators; TENS devices, electrical acupuncture. Thermal energy - cryosurgery equipment; heat exchangers. Mechanical energy - powered dermatomes; drills and dental hand pieces. Light - phototherapy for skin treatment and for neonatal care. Sound - hearing aids. | 4.2(1) Subject to subclause (2)… to be used to administer energy to a patient, or exchange energy to or from a patient Class IIa |
Kinetic energy - lung ventilators. Thermal energy - infant incubators; warming blankets for unconscious patients; blood warmers; heat exchangers used in intensive care. Electrical energy - high-frequency electrosurgical generators, electrocautery, external defibrillators, electroconvulsive therapy equipment. Coherent light - surgical lasers. Ultrasound - lithotripters; physiotherapy ultrasound devices. Ionising radiation - radioactive sources for after-loading therapy; therapeutic cyclotrons; linear accelerators; therapeutic X-ray sources. | 4.2(2) If the device is of a kind such that the administration or exchange of energy occurs in a potentially hazardous way, having regard to the nature, density and site of application of the energy Class IIb. |
| An active device that determines the treatment parameters used by a physiotherapy ultrasound device. | 4.2(3) To be used to control or monitor, or directly influence, the performance of an active medical device for therapy of the kind mentioned in subclause (2) Class IIb. |
Rule 4.4 - Active medical devices intended to administer or remove medicines, etc from a patient’s body
Administering and removing medicines or other substances from a patient’s body
This rule applies to active medical devices that are to be used to:
- administer medicine, body liquids or other substances to a patient, or
- remove medicine, body liquids or other substances from a patient.
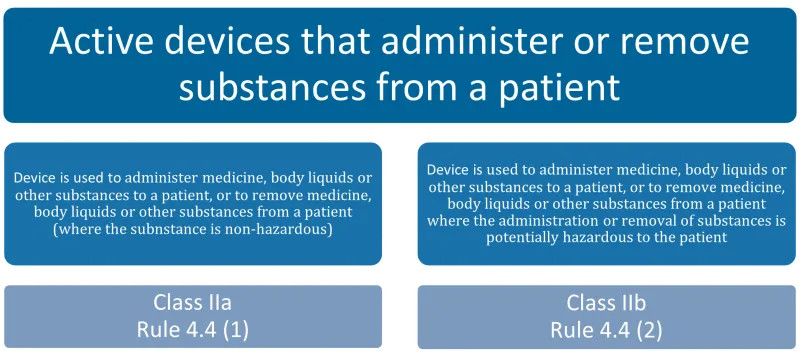
This chart categorises active devices that administer or remove substances from a patient into two types, each with its corresponding classification and rule. The categories are:
- Devices used to administer medicine, body liquids or other substances to a patient, or to remove these from a patient, where the substance is non-hazardous: Class IIa, Rule 4.4 (1)
- Devices used to administer medicine, body liquids or other substances to a patient, or to remove these from a patient, where the administration or removal of substances is potentially hazardous to the patient: Class IIb, Rule 4.4 (2).
| Examples | Rule and classification |
|---|---|
| Suction equipment; feeding pumps; standard nebulisers; jet injectors for vaccination | 4.4(1) Subject to subclause (2)… to be used to administer or remove medicine, body liquids or other substances to or from a patient Class IIa. |
Pressure regulators for medical gases Medical-gas mixers Nebulisers where the failure to deliver the appropriate dosage form could be hazardous Infusion pumps Ventilators Anaesthesia machines Anaesthetic vaporisers Dialysis equipment Blood pumps for heart–lung machines Hyperbaric chambers Ultrasonic (fast-track) nebulisers | 4.4(2) If the device is of a kind such that the administration or removal of medicine, body liquids or other substances is potentially hazardous, having regard to the nature of the substances involved, the part of the patient’s body concerned, and the characteristics of the device Class IIb. |
Rule 4.8 - Providing therapy through the provision of information
Information-based therapy
This rule applies to devices intended to provide therapy to a person through the provision of information to the person. The classification depends on the seriousness of the risk of harm to a person.
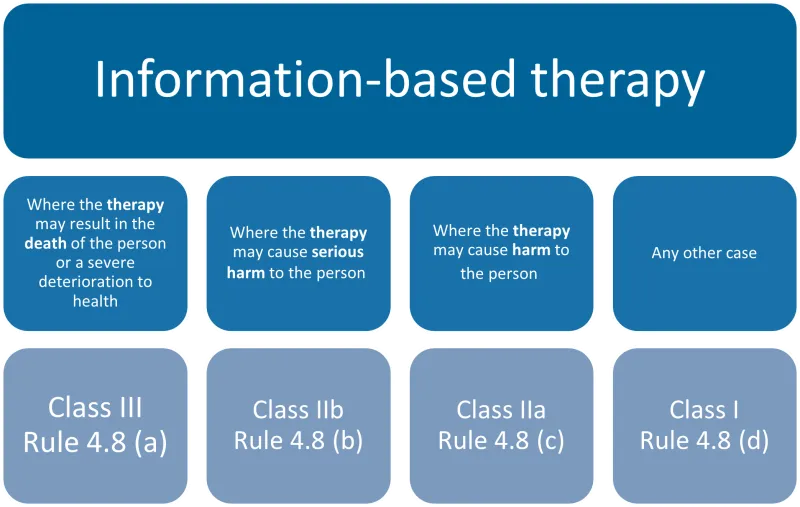
This chart categorises information-based therapy devices into four types based on the potential harm level, each with its corresponding classification and rule:
- Where the therapy may result in the death of the person or a severe deterioration to health: Class III, Rule 4.8 (a)
- Where the therapy may cause serious harm to the person: Class IIb, Rule 4.8 (b)
- Where the therapy may cause harm to the person: Class IIa, Rule 4.8 (c)
- Any other case: Class I, Rule 4.8 (d).
| Examples | Rule and classification |
|---|---|
| An app intended to provide cognitive behavioural therapy to a patient for treating bipolar disorder that does not reference an established clinical practice guideline. | 4.8(c) In the case of therapy that may cause harm to the person and where neither paragraph (a) nor (b) applies Class IIa. |
Note: software that is a digital mental health tool (including a cognitive behaviour therapy tool) based on established clinical practice guidelines referenced and displayed in the software in a manner reviewable by the user is not a medical device as per the Therapeutic Goods (Excluded Goods) Determination 2018.
Rule 5.4
Recording patient images and anatomical models
Rule 5.4 will apply to medical devices,1 including software-based medical devices, for diagnosis and/or investigation and are intended to:
- record patient images
- anatomical models (virtual or physical), or
- intended to generate virtual anatomical models.
Refer to the section titled Recording patient images for the rules on medical devices intended to be used to record patient images for diagnosis, monitoring, or investigation.
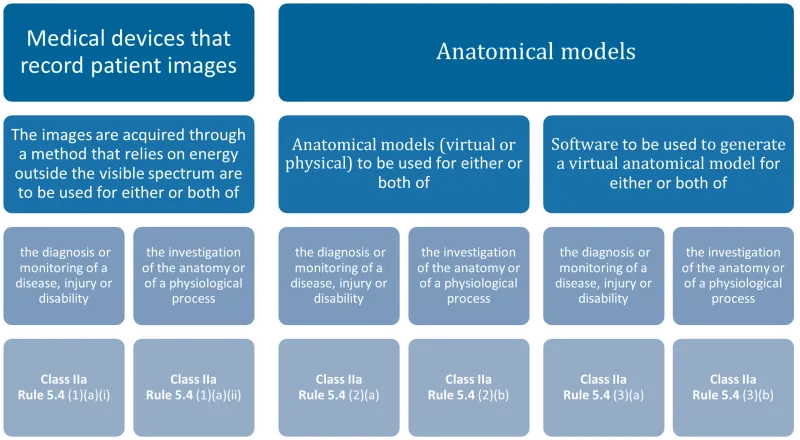
This chart categorises medical devices into two main groups: devices that record patient images and anatomical models. Each group is further divided based on their use and method:
- Medical devices that record patient images:
- Images acquired through energy outside the visible spectrum, used for diagnosis or monitoring of disease, injury or disability: Class IIa, Rule 5.4 (1)(a)(i)
- Images acquired through energy outside the visible spectrum, used for investigation of anatomy or physiological process: Class IIa, Rule 5.4 (1)(a)(ii)
- Anatomical models:
- Virtual or physical models used for diagnosis or monitoring of disease, injury or disability: Class IIa, Rule 5.4 (2)(a)
- Virtual or physical models used for investigation of anatomy or physiological process: Class IIa, Rule 5.4 (2)(b)
- Software used to generate virtual anatomical models for diagnosis or monitoring of disease, injury or disability: Class IIa, Rule 5.4 (3)(a)
- Software used to generate virtual anatomical models for investigation of anatomy or physiological process: Class IIa, Rule 5.4 (3)(b).
| Examples | Rule and classification |
|---|---|
Software intended to record images captured from an ultrasound device and intended to be used to diagnose a torn ligament. Note, if the software captured and analysed an image to provide a specific diagnosis of a disease or condition, then rule 4.5 would apply. | 5.4(1) To record patient images that are to be used for either or both of the diagnosis and monitoring of a disease, injury or disability; and the investigation of the anatomy or of a physiological process; and the images are to be acquired through a method that relies on energy outside the visible spectrum Class IIa. |
| Examples | Rule and classification |
|---|---|
| A three-dimensional model produced from magnetic resonance imaging data that is intended to help a surgeon plan facial reconstruction surgery. | 5.4(2) An anatomical model (whether physical or virtual) to be used for either or both of the diagnosis or monitoring of a disease, injury or disability; or the investigation of the anatomy or of a physiological process Class IIa. |
| Examples | Rule and classification |
|---|---|
| Software intended to generate a three-dimensional virtual anatomical model from patient scans, to assist a health professional in diagnosing cardiovascular disease. | 5.4(3) To be used to generate a virtual anatomical model that is to be used for either or both of the diagnosis or monitoring of a disease, injury or disability; and the investigation of the anatomy or of a physiological process Class IIa. |
Rule 5.7 - Active implantable medical devices
Implantable devices
This rule applies to:
- active implantable medical devices
- implantable accessories to active implantable medical devices
- active medical devices to be used to control or monitor, or directly influence, the performance of active implantable medical devices.
Note that from 25 November 2021 active implantable medical devices will be reclassified from Class AIMD to Class III. See Reclassification of Active Implantable Medical Devices (AIMD) for more information.
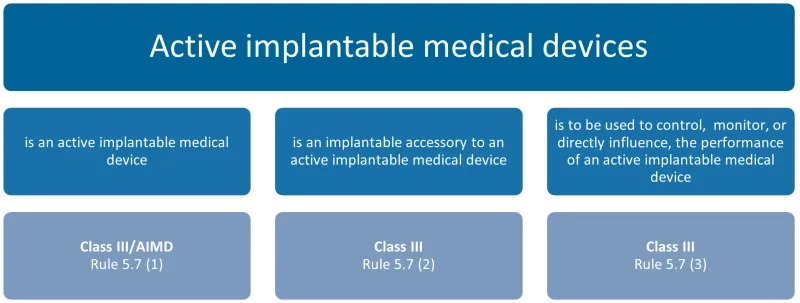
This chart categorises active implantable medical devices into three types, each with its corresponding classification and rule:
- Is an active implantable medical device: Class III/AIMD, Rule 5.7 (1)
- Is an implantable accessory to an active implantable medical device: Class III, Rule 5.7 (2)
- Is to be used to control, monitor, or directly influence the performance of an active implantable medical device: Class III, Rule 5.7 (3).
| Examples | Rule and classification |
|---|---|
| Pacemakers. | 5.7(1) An active implantable medical device - Class AIMD. Class III from 25 November 2021. |
| Electrode leads associated with pacemakers, defibrillators, nerve stimulators. | 5.7(2) An implantable accessory to an active implantable medical device - Class III. |
| Clinician’s programming device for pacemakers, patient control devices for nerve stimulation devices. | 5.7(3) An active medical device… to be used to control or monitor, or directly influence, the performance of an active implantable medical device - Class III. |
Rule 4.1
General rule for any other active medical device
This rule classifies any active medical devices, including software-based medical devices, not covered by any other rule as Class I (i.e., this is the default rule if no other rule applies to your device).
| Examples | Rule and classification |
|---|---|
| Examination lights; surgical microscopes; dental curing lights; surgical microscopes with recording functionality. | 4.1 An active medical device is classified as Class I, unless the device is classified at a higher level under another clause - Class I. |
Footnotes
- The majority of devices will be active medical devices; however, Rule 5.4 applies irrespective of whether a device is an active medical device or not.
Page history
Title changed from 'Classification of active medical devices (including software-based medical devices)' to 'Classifying active medical devices in Australia (including software-based medical devices)' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
V1.0 Original publication
Title changed from 'Classification of active medical devices (including software-based medical devices)' to 'Classifying active medical devices in Australia (including software-based medical devices)' as part of migration to new 'Guidance' content type:
- Consistent ‘Purpose’ heading.
- ‘Legislation’ section to clearly show which laws the Guidance relates to.
- ‘Page history’ section replaces document version history.
- New page navigation features.
- Updated page summaries.
- Complex images include long descriptions.
- New ‘Save as PDF’ feature.
V1.0 Original publication

