Summary
From 1 January 2019 to 31 December 2019, the Therapeutic Goods Administration (TGA) conducted 10 pharmacovigilance inspections of Australian medicine sponsors, in accordance with the Pharmacovigilance Inspection Program.
10 routine pharmacovigilance inspections were completed in 2019. There were no 'for-cause' inspections or re-inspections.
Inspections identified:
- 1 critical deficiencies
- 41 major deficiencies
- 36 minor deficiencies.
The number and grading of deficiencies by pharmacovigilance topic area, is described in Table 1 (see Appendix I for more detail on each pharmacovigilance topic area). All deficiencies have either been rectified or have a Corrective and Preventative Action (CAPA) plan in place and are in the process of being resolved.
An overall improvement in sponsor compliance with pharmacovigilance legislation and guidelines was observed in this reporting period compared to the first period (1 September 2017 and 31 December 2018) which is reflected, in particular, by the lower no. of major deficiencies (see Comparison of inspection deficiencies over time).
| Topic area | Critical (1) | Major (41) | Minor (36) |
|---|---|---|---|
| Collection and collation of adverse drug reactions | 1 | 5 | 4 |
| Management of adverse drug reactions | - | 3 | 6 |
| Reporting serious adverse drug reactions | - | 8 | - |
| Ongoing safety evaluation | - | 2 | 4 |
| Management of significant safety issues | - | 10 | - |
| Management of reference safety information | - | 8 | 2 |
| Post-approval commitments | - | - | 7 |
| Quality management system | - | 3 | 8 |
| Australian Pharmacovigilance Contact Person & Qualified Person Responsible for Pharmacovigilance in Australia (QPPVA) | - | 2 | 5 |
Background
The TGA Pharmacovigilance Inspection Program (PVIP) commenced on 1 September 2017 following a successful pilot inspection program in 2015-16.
The PVIP aims to strengthen and broaden the TGA's post-market monitoring activities and protect public health by ensuring the continued safety of medicines included in the Australian Register of Therapeutic Goods (ARTG).
Pharmacovigilance inspections allow the TGA to help sponsors meet their pharmacovigilance obligations and maintain effective and robust pharmacovigilance systems. The inspections assess sponsor compliance with applicable Australian pharmacovigilance regulations and guidelines, in particular, the:
- Therapeutic Goods Act 1989 (section 28(5e), 28(5)(ca), 28(2B), 28(3), 29A and 29AA)
- Therapeutic Goods Regulations 1990 (Regulation 15A)
- Pharmacovigilance responsibilities of medicine sponsors Australian recommendations and requirements (v2.1, June 2018)
- Conditions, standard and specific, applying to registered or listed therapeutic goods (section 28 of the Act).
The TGA applies a risk-based approach to scheduling pharmacovigilance inspections. This takes into account risk factors related to the sponsor, including their products, pharmacovigilance system and compliance history. The TGA also reviews data provided through the PVIP Risk Assessment Survey to help plan and schedule inspections.
This metrics report contains data from 10 inspections conducted from 1 January 2019 to 31 December 2019. The report provides a high-level overview of inspection deficiencies including a comparison of deficiencies identified in the first reporting period, to assist sponsors with improving their pharmacovigilance systems and preparing for pharmacovigilance inspections. All information has been de-identified.
Inspections conducted
From 1 January 2019 to 31 December 2019, the TGA conducted 10 routine pharmacovigilance inspections of Australian medicine sponsors (see Appendix II for types of inspections). There were no 'for-cause' inspections or re-inspections, however sponsors were selected in accordance with the TGA's risk-based approach to scheduling inspections.
Nine inspections were conducted over three days and one inspection was conducted over two days. Six inspections were conducted onsite, at the physical premises of the Australian sponsor, and four inspections were conducted remotely via video or teleconference, with no TGA inspectors attending company offices in person.
A variety of medicine sponsors were inspected during this period, including large and small innovator companies, as well as sponsors of generic medicines, vaccines, over-the-counter and complementary medicines.
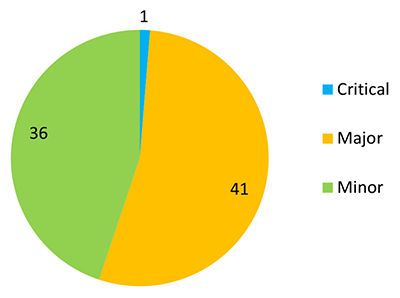
Deficiencies identified during inspections were graded as critical, major or minor (see Appendix III for definitions of inspection gradings). From the 10 pharmacovigilance inspections conducted during the reporting period, the TGA identified:
- 1 critical deficiency
- 41 major deficiencies
- 36 minor deficiencies
A reported deficiency may have comprised of multiple separate findings, grouped according to a high-level legislative requirement or according to a cumulative pharmacovigilance impact.
The deficiencies are discussed in the next section (see Inspection findings).
Inspection findings
In this section: Critical findings | Major findings | Minor findings
Critical deficiencies
One critical deficiency was identified from 10 inspections conducted during the reporting period.
The critical deficiency was categorised under the pharmacovigilance topic area: collection and collation of adverse drug reactions (see Appendix I) and comprised of multiple findings that contributed to a failure to collect and collate adverse drug reactions, including serious adverse drug reactions that warranted reporting to the TGA within 15 calendar days.
A critical grading was applied to the deficiency because it represented a serious violation of applicable pharmacovigilance legislation and guidelines, and posed a potential risk to public health.
Following the inspection, the sponsor developed a CAPA plan that addressed the critical deficiency and proposed actions to mitigate the risk of reoccurrence. CAPA commitments are closely monitored by the TGA until they are fully implemented. At the time of publishing this report, most CAPA actions have been completed.
Sponsors are reminded that they need to be able to identify and collect all information related to the safety of their medicine, from all possible sources, to effectively monitor the medicine's safety. All serious Australian adverse drug reactions must be reported to the TGA within 15 calendar days of first receipt.
Major deficiencies
41 major deficiencies were identified from 10 inspections conducted during the reporting period with at least three major deficiencies identified in every inspection. The number of major deficiencies identified per each inspection ranged between three and six (see Figure 2).
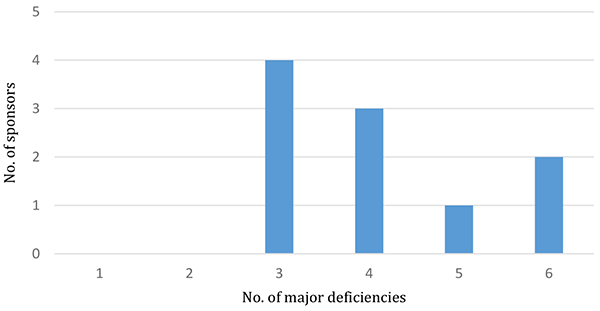
Figure 2 in table format
| Number of major deficiencies | Number of sponsors |
|---|---|
| 3 | 4 |
| 4 | 3 |
| 5 | 1 |
| 6 | 2 |
Major deficiencies by topic area
The 41 major deficiencies identified during the reporting period have been grouped by overarching topics across the pharmacovigilance system. Each topic area is also made up of various sub-topics (see Appendix I).
The proportion of major deficiencies by pharmacovigilance topic area is described in Figure 3.
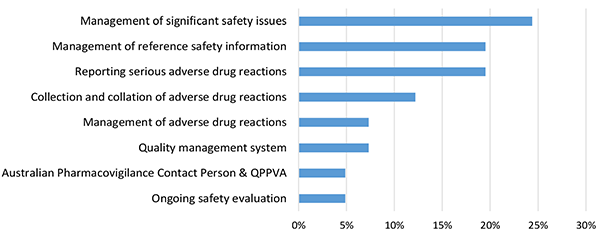
Figure 3 in table format
| Topic area | Proportion of major deficiencies |
|---|---|
| Management of significant safety issues | 24.39% |
| Management of reference safety information | 19.51% |
| Reporting serious adverse drug reactions | 19.51% |
| Collection and collation of adverse drug reactions | 12.20% |
| Management of adverse drug reactions | 7.32% |
| Quality management system | 7.32% |
| Australian Pharmacovigilance Contact Person & QPPVA | 4.88% |
| Ongoing safety evaluation | 4.88% |
Summary of major deficiencies
The topic with the highest proportion of major deficiencies in the reporting period was the management of significant safety issues (24%), followed by management of reference safety information (19%) and reporting serious adverse drug reactions (19%). These deficiencies are discussed in more detail in the next section (see Common areas of deficiencies).
Minor deficiencies
36 minor deficiencies were identified from 10 inspections conducted during the reporting period with at least one minor deficiency identified in every inspection. The number of minor deficiencies identified per inspection ranged between one and six (see Figure 4).
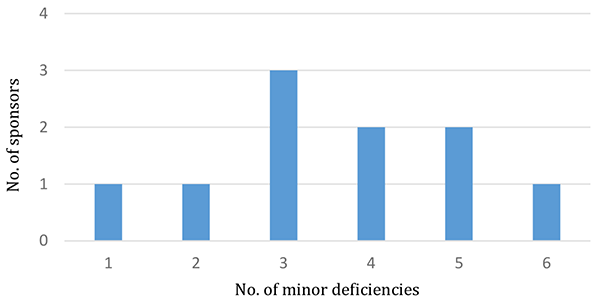
Figure 4 in table format
| Number of minor deficiencies | Number of sponsors |
|---|---|
| 1 | 1 |
| 2 | 1 |
| 3 | 3 |
| 4 | 2 |
| 5 | 2 |
| 6 | 1 |
Minor deficiencies by topic area
The 36 minor deficiencies identified during the reporting period have been grouped by overarching topics across the pharmacovigilance system. Each topic area is also made up of various sub-topics (see Appendix I).
The proportion of minor deficiencies by pharmacovigilance topic area is described in Figure 5.
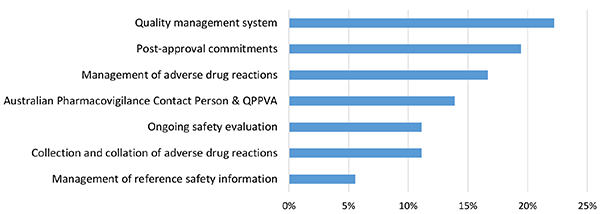
Figure 5 in table format
| Topic area | Proportion of minor deficiencies |
|---|---|
| Quality management system | 22.22% |
| Post-approval commitments | 19.44% |
| Management of adverse drug reactions | 16.67% |
| Australian Pharmacovigilance Contact Person & QPPVA | 13.89% |
| Ongoing safety evaluation | 11.11% |
| Collection and collation of adverse drug reactions | 11.11% |
| Management of reference safety information | 5.56% |
Summary of minor deficiencies reported during the period
The topic with the highest proportion of minor deficiencies in the reporting period was the quality management system (22%), followed by post-approval commitments (19%) and management of adverse drug reactions (17%). These deficiencies are discussed in more detail in the next section (see Common areas of deficiencies).
Common areas of deficiencies
In this section: Collection and collation of adverse drug reactions | Management of significant safety issues | Management of reference safety information | Reporting serious adverse drug reactions | Management of adverse drug reactions | Quality management system | Australian pharmacovigilance contact person and Qualified Person for Pharmacovigilance in Australia (QPPVA) | Post-approval commitments
Collection and collation of adverse drug reactions
A deficiency in collection and collation of adverse drug reactions was observed in every inspection conducted during the reporting period and represented the only critical deficiency, as well as 12% of major deficiencies and 11% of minor deficiencies. Deficiencies were identified in the following processes:
- Collecting safety information from medical information enquiries, product quality complaints, company-sponsored websites and social media, local and international medical literature, internal company departments, business partners and post-registration programs (e.g. patient support programs, product familiarisation programs, market research programs etc.)
- Reconciling safety information with all sources
Sponsors should maintain a pharmacovigilance system that allows them to identify, collect and collate all information related to safety of their medicine from all possible sources. Sponsors should also exercise due diligence and develop procedures to collate accurate and complete reports of adverse drug reactions.
Management of significant safety issues
Deficiency in the management of significant safety issues was observed in every inspection conducted during the reporting period and represented the highest proportion of major deficiencies (24%). This deficiency comprised of multiple findings related to a failure to identify significant safety issues or a failure to report significant safety issues to the TGA within 72 hours of first awareness.
Sponsors are reminded that a significant safety issue is a new safety issue or validated signal considered by you in relation to your medicine that requires urgent attention of the TGA. This may be because of the seriousness and potential major impact on the benefit-risk balance of the medicine and/or on patient or public health, which could warrant prompt regulatory action and/or communication to patients and healthcare professionals. The TGA expects sponsors to use clinical judgement when determining whether a safety issue is significant.
Therefore, internally validated signals that meet the definition of a significant safety issue must be reported to the TGA within 72 hours of first awareness. Sponsors should have robust procedures in place to ensure that validated signals are promptly assessed against the Australian pharmacovigilance guidelines to ensure compliance with regulatory reporting timeframes.
The TGA also considers significant safety issues to include safety-related actions taken by comparable international regulatory agencies, such as the addition or modification of a contraindication, warning or precaution statement to the product information.
Therefore, sponsors should also have procedures in place to regularly screen worldwide medical literature and information published by comparable foreign regulatory agencies, for safety-related information related to their medicine. Publications containing information that meet the definition of a significant safety issue must be reported to the TGA within 72 hours.
Management of reference safety information
Deficiency in the management of reference safety information of any grading was observed in every inspection conducted during the reporting period and represented the second highest proportion (19%) of major deficiencies, along with reporting serious adverse drug reactions. This deficiency comprised of multiple findings related to delays in updating the Australian Product Information (PI), Consumer Medicine Information (CMI), product packaging leaflets and product labelling, with new or revised safety information.
Sponsors are reminded that the TGA expects all safety-related requests to update the Australian PI, are submitted within 6 months from the date that it was first decided that a variation is required. Sponsors of generic medicines are reminded that it is a specific condition of registration to submit an application to the TGA within one month of a safety-related update to the innovator PI, to align their Australian PI.
Sponsors must ensure that safety-related changes to the Australian PI are reflected in the CMI, where applicable. The updated PI and CMI must be uploaded to the TGA website within two weeks of TGA approval. Company-sponsored material (e.g. educational or promotional items) impacted by safety-related changes to the Australian PI should also be updated within a timely manner, following TGA approval.
Sponsors should also ensure that relevant company personnel are promptly notified of important safety-related changes to the Australian PI following TGA approval, to ensure the safe use of medicines by patients and healthcare professionals.
Reporting serious adverse drug reactions
Deficiency in reporting serious adverse drug reactions was observed in 8 out of 10 inspections conducted during the reporting period, and represented the second highest proportion (19%) of major deficiencies, along with the management of reference safety information.
Sponsors are reminded that all serious adverse drug reactions must be reported to the TGA within 15 calendar of first receipt by any company personnel, including any 3rd party personnel conducting activities on behalf of the sponsor. Sponsors should confirm that all company personnel are trained in their pharmacovigilance reporting obligations and that robust procedures are in place to ensure that all safety information is reported promptly, to allow for compliance with the 15-day reporting timeframe. Sponsors should periodically audit their pharmacovigilance agreements to ensure that business partners or contracted personnel are complying with their pharmacovigilance reporting obligations.
Management of adverse drug reactions
Deficiency in the management of adverse drug reactions of any grading was observed in 9 out of 10 inspections conducted during the reporting period, and represented 7% of major deficiencies and 17% of minor deficiencies.
Sponsors are reminded that the seriousness of an adverse drug reaction report is linked to the seriousness of the reported adverse reaction. It is important that seriousness assessments are an independent process to medical evaluation, causality and validity of the case and are based on the adverse reaction alone. Where outcomes or treatment information (e.g. hospitalisation) is not available, a conservative approach should always be taken.
The TGA also expects sponsors to make reasonable effort to follow-up, where possible, on all adverse drug reaction reports received, to obtain missing information and detailed supplementary information significant to the clinical evaluation of the case. For reports involving pregnancies, where the embryo or foetus could have been exposed to a medicine, the TGA expects sponsors to make reasonable attempts to follow up all individual cases and collect information on the outcome of the pregnancy and development of the child after birth.
For reports received directly from patients, sponsors should seek informed consent to contact the patient's medical practitioner for medical confirmation of the adverse reaction or directly request the patient to provide relevant medical documentation.
Sponsors are reminded that patients can also provide valuable information on their case if consent to contact the patient's medical practitioner is denied. If patients are not comfortable with providing their report to the company, then patients should be encouraged to report their adverse drug reaction to the TGA, by providing the contact details for reporting.
Quality management system
Deficiency in the quality management system of any grading was observed in every inspection conducted during the reporting period, and represented the highest proportion (22%) of minor deficiencies and 7% of major deficiencies.
Sponsors should establish a quality management system that supports their pharmacovigilance system to meet their Australian pharmacovigilance requirements. These may include, but are not limited to, standard operating procedures for:
- collecting, processing and reporting adverse drug reactions
- management of significant safety issues
- management of reference safety information
- pharmacovigilance training
- management and retention of pharmacovigilance records
- audit and deviation management
Australian pharmacovigilance contact person and Qualified Person for Pharmacovigilance in Australia (QPPVA)
A deficiency of any grading, related to the role of the Australian pharmacovigilance contact person and/or the QPPVA, was observed in 7 out of 10 inspections conducted during the reporting period, and represented 5% of major deficiencies and 14% of minor deficiencies. Deficiencies in this topic area were related to:
- a failure to notify the TGA with the name and contact details of the Australian pharmacovigilance contact person within 15 calendar days of the first medicine's entry in the ARTG, or any subsequent updates to this position (e.g. new name or updated contact details)
- inadequate oversight of the pharmacovigilance system
Sponsors are reminded that they must nominate a pharmacovigilance contact person in Australia who will be responsible for fulfilling their pharmacovigilance reporting requirements. The nominated pharmacovigilance contact person must reside in Australia and should have a sound understanding of Australian pharmacovigilance reporting requirements. The Australian pharmacovigilance contact person can be nominated, or their details updated, through the TGA Business Services Portal.
Sponsors should also have a qualified person responsible for pharmacovigilance undertakings in Australia (i.e. the QPPVA). This person may be different to the Australian pharmacovigilance contact person, although ideally they are the same person.
The QPPVA should ensure that the sponsor has an effective pharmacovigilance system in place to comply with Australian pharmacovigilance requirements, and have an adequate understanding of Australian and global pharmacovigilance processes, in order to have effective oversight of the entire pharmacovigilance system.
Post-approval commitments
Deficiency in compliance with post-approval commitments was observed in 7 out of 10 inspections conducted during the reporting period, and represented the second highest proportion (19%) of minor deficiencies.
Sponsors are reminded that the preparation and timely submission of Periodic Safety Update Reports, maintenance of Risk Management Plans and compliance with Risk Management Plan commitments are stipulated as a condition of registration.
Comparisons of inspection findings over time
Since the commencement of the Pharmacovigilance Inspection Program, on 1 September 2017, to 31 December 2019, the TGA has conducted 20 pharmacovigilance inspections of Australian medicine sponsors. Ten inspections were conducted in the first reporting period, between 1 September 2017 and 31 December 2018, and ten inspections were conducted in the current (second) reporting period, between 1 January 2019 and 31 December 2019. All inspections conducted in the first reporting period were conducted in the 2018 calendar year.
A comparison of the no. of deficiencies between the current (second) reporting period and the first reporting period, by pharmacovigilance topic area, is described below (see Figure 6).
It should be noted that the criteria for certain metric items reported in this period have changed from the first period. Therefore, comparisons between the number of deficiencies observed in each reporting period, related to the management of adverse drug reactions, the quality management system and post-approval commitments should be interpreted with caution.
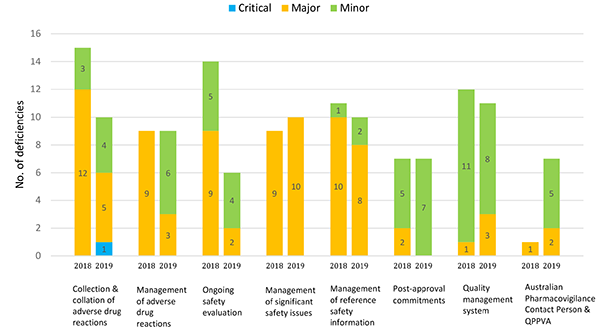
Figure 6 in table format
| Topic area | Critical deficiencies 2018 | Critical deficiencies 2019 | Major deficiencies 2018 | Major deficiencies 2019 | Minor deficiencies 2018 | Minor deficiencies 2019 |
|---|---|---|---|---|---|---|
| Collection and collation of adverse drug reactions | 0 | 1 | 12 | 5 | 3 | 4 |
| Management of adverse drug reactions | 0 | 0 | 9 | 3 | 0 | 6 |
| Ongoing safety evaluation | 0 | 0 | 9 | 2 | 5 | 4 |
| Management of significant safety issues | 0 | 0 | 9 | 10 | 0 | 0 |
| Management of reference safety information | 0 | 0 | 10 | 8 | 1 | 2 |
| Post-approval commitments | 0 | 0 | 2 | 0 | 5 | 7 |
| Quality management system | 0 | 0 | 1 | 3 | 11 | 8 |
| Australian Pharmacovigilance Contact Person & QPPVA | 0 | 0 | 1 | 2 | 0 | 5 |
In the current reporting period, the total no. of deficiencies related to case collection & collation, and ongoing safety evaluation, is less than the first period. For case collection and collation, the no. of major deficiencies was less than half the no. observed in the first period, however one critical deficiency was identified in this reporting period. Similarly, for ongoing safety evaluation, the total no. of deficiencies was less half the number observed in the first period, with the most significant decrease observed in those deficiencies with a major grading.
The total no. of deficiencies related to management of adverse drug reactions, management of significant safety issues, management of reference safety information, post-approval commitments and the quality management system was comparable between the current and first reporting period.
However, for the management of adverse drug reactions, the no. of major deficiencies was one-third of the no. observed in the first period. It should be noted that deficiencies from the first reporting period included findings related to reporting serious adverse drug reactions, which is a separate metric item (see Appendix I) in the current reporting period (not shown in Figure 6) and accounted for a large proportion of major deficiencies for this topic. Therefore, the number of major deficiencies related to the management of adverse drug reactions may be overrepresented in the first reporting period.
Similarly, no major deficiency was identified for post-approval commitments in the current reporting period compared with two major deficiencies in the first period. It should be noted that deficiencies from the first reporting period did not include findings related to Risk Management Plans and therefore, the no. of deficiencies related to compliance with post-approval commitments in the first period, may be underrepresented.
The total no. of deficiencies for the quality management system was comparable between reporting periods. However, it should be noted that deficiencies from the first reporting period did not include findings related to the management and retention of pharmacovigilance records and therefore the no. of deficiencies related to the quality management system in the first period may be underrepresented.
Deficiencies related to role of the Australian pharmacovigilance contact person or the QPPVA is the only topic area which observed an increase in the total no. of deficiencies, increasing from one major deficiency in the first reporting period, to two major and five minor deficiencies in the current period.
Appendix I: Pharmacovigilance topic areas
| Topic area | Sub-topic |
|---|---|
| Collection and collation of adverse drug reactions | Spontaneous sources of safety data, including medical information, product quality complaints, medical literature, company personnel (e.g. sales representatives, social/digital media etc.) |
| Solicited sources of safety data, including patient support or market research programs, observational studies etc. | |
| Safety data exchange agreements | |
| Management of adverse drug reactions | Case processing, including data entry, coding, causality and seriousness assessment, and follow-up |
| Reporting serious adverse drug reactions | Reporting serious adverse drug reactions within 15 calendar days |
| Ongoing safety evaluation | Signal detection and management |
| Production of Periodic Safety Update Reports | |
| Management of significant safety issues | Identifying significant safety issues |
| Reporting significant safety issues within 72 hours | |
| Management of reference safety information | Maintenance of core safety information |
| Maintenance of Australian Product Information, Consumer Medicine Information, product packaging leaflets and product labelling | |
| Maintenance of safety-related information in company-sponsored material (e.g. educational or promotional items) | |
| Communication of updated safety-related information to internal and external stakeholders | |
| Post-approval commitments | Submission of Periodic Safety Update Reports |
| Maintenance and submission of Risk Management Plans | |
| Compliance with Risk Management Plan commitments | |
| Compliance with other conditions of registration | |
| Quality management system | Management and retention of pharmacovigilance records |
| Pharmacovigilance training | |
| Management of pharmacovigilance procedures | |
| Audit and deviation management | |
| Australian Pharmacovigilance Contact Person & Qualified Person Responsible for Pharmacovigilance in Australia (QPPVA) | Notification of the Australian Pharmacovigilance Contact Person within 15 calendar days |
| QPPVA oversight of the pharmacovigilance system |
Appendix II: Types of inspections
*excerpt from page 10-11 of the Pharmacovigilance inspection program: Guidance for medicine sponsors (Version 1.0, September 2017). Please refer to the full guidance for information on all types of inspections.
Please note the TGA is referred to as 'we' or 'us', and sponsors as 'you'.
Routine inspections
Routine pharmacovigilance inspections are scheduled as part of the inspection program. There is no specific trigger for these inspections, although we take a risk-based approach to prioritising them. These inspections are usually system-related inspections, but one or more products may be selected as examples to verify the implementation of the system and provide practical evidence of its functioning and compliance.
'For cause' inspections
'For cause' inspections are undertaken in response to specific triggers where a pharmacovigilance inspection is the appropriate way to examine the issues. 'For cause' inspections generally focus on specific aspects of the sponsor's pharmacovigilance system or examine identified compliance issues and their impact on a specific product. However, we may also inspect the sponsor's entire pharmacovigilance system as a result of a trigger. Significant public health concerns or identified noncompliance are expected to be the most common triggers.
Re-inspections
We may re-inspect the sponsor's pharmacovigilance system as part of our routine inspection program. We prioritise re-inspections by assessing risk factors. If a previous inspection identified a high level of compliance this may increase the time between re-inspections. More frequent re-inspections may occur:
- where we have identified significant noncompliance
- to verify sponsors have taken action to address inspection findings
- to evaluate the sponsor's ongoing compliance with their obligations and evaluate changes to their pharmacovigilance system
- when a previous inspection finds a sponsor had failed to take appropriate corrective and preventative action in response to prior inspections.
Remote inspections
These are pharmacovigilance inspections of the sponsor's premises (or the premises of a firm contracted to help fulfil the sponsor's pharmacovigilance activities) that we perform remotely using communication technology such as the internet or video/tele conferencing. For example, where key sites for pharmacovigilance activities are located outside Australia or a third-party service provider is not available at the inspection site, it may be feasible to interview relevant staff and review documentation via remote access. If the remote inspection reveals issues that require onsite inspection, or the inspection objectives could not be met remotely, we may visit the inspection site.
Appendix III: Definition of inspection gradings
*excerpt from page 21 of the Pharmacovigilance inspection program: Guidance for medicine sponsors (Version 1.0, September 2017)
Critical deficiency:
A deficiency in pharmacovigilance systems, practices or processes that adversely affects the rights, safety or well-being of patients or that poses a potential risk to public health or that represents a serious violation of applicable legislation and guidelines.
Deficiencies classified as critical may include a pattern of deviations classified as major.
A critical deficiency also occurs when a sponsor is observed to have engaged in fraud, misrepresentation or falsification of data.
Major deficiency:
A deficiency in pharmacovigilance systems, practices or processes that could potentially adversely affect the rights, safety or well-being of patients or that could potentially pose a risk to public health or that represents a violation of applicable legislation and guidelines.
Deficiencies classified as major may include a pattern of deviations classified as minor.
Minor deficiency:
A deficiency in pharmacovigilance systems, practices or processes that would not be expected to adversely affect the rights, safety or well-being of patients.
AA deficiency may be minor either because it is judged as minor or because there is insufficient information to classify it as major or critical.
Note:
- Deficiencies are classified by the assessed risk level and may vary depending on the nature of medicine. In some circumstances an otherwise major deficiency may be categorised as critical.
- A deficiency reported after a previous inspection and not corrected may be given higher classification.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Orginal publication | Risk Management Section, Pharmacovigilance and Special Access Branch | May 2020 |

