Creating prescription medicine labels that meet requirements
Tips for sponsors, distributors and retailers on creating prescription medicine labels that are compliant with Therapeutic Goods Order No. 91 - Standard for labels of prescription and related medicines (TGO 91).
Recently published
This page was published on [date_placeholder].
Recently updated
This page was updated on [date_placeholder]. See page history for details.
Purpose
This guidance is for sponsors, distributors and retailers responsible for designing and updating prescription medicine product labels.
Legislation
Medicine name on the main label
Requirements
The main label is the portion where the name of the medicine is more or most conspicuously shown (Section 6 of TGO 91). Where the name of the medicine is equally conspicuous on 2 or more portions of a label, each portion is the main label and all information required on the main label, must be repeated on all portions.
Information that must be included on main labels is defined in Section 9 of TGO 91. Additionally, the main label must include the AUST R number (Therapeutic Goods Regulations 1990) and relevant signal heading relating to the relevant scheduling classification (Poisons Standard) for the medicine.
Further details are outlined in Guidance on TGO 91 and TGO 92 (see Section 1.7).
Question
What is wrong with the following example?

Technical layout diagram showing 3 panels of a medicine package design with annotations.
The layout includes:
- Top panel: 'Medicine name' with 'active ingredient 50 mg' below it.
- Middle panel (main): Contains multiple elements:
- Vertical text on left reading 'Medicine name active ingredient 50 mg'
- Warning text 'PRESCRIPTION ONLY MEDICINE' and 'KEEP OUT OF REACH OF CHILDREN'
- 'Medicine name' and dosage information
- '10 tablets' specification
- 'AUST R XXXXX' registration number
- Right side elements including a red prohibition symbol, blue '50 mg' label, decorative blue balloon-like icon, and 'Sponsor' text
- Bottom panel: Repeats 'Medicine name' and 'active ingredient 50 mg'
- A red annotation note in the top-left states 'Medicine name is equally prominent. All panels are main labels.'
All text elements are clearly laid out in a structured format with consistent spacing and hierarchy, almost following pharmaceutical packaging standards.
Answer
The medicine name is equally conspicuous (that is, same text size and formatting) on all 4 portions of the label shown. Therefore, all 4 portions are main labels and must include the AUST R number, the signal heading and caution statements, and all ‘main label’ information stated in Section 9 of TGO 91, including the name of the dosage form and the quantity of the medicine.
Solution
Increase the prominence of the medicine name on the main label. This makes your main label obvious by using different text sizes for the medicine name. You could increase the text size of the medicine name on the main label or decrease the text size on other portions.
If you want 2 (or more panels) to have equally prominent medicine names, then each panel must include all ‘main label’ information.

A pharmaceutical package layout divided into three panels showing approved design elements:
- Top panel: Contains 'Medicine name' with 'active ingredient 50 mg' underneath.
- Middle panel (main section):
- Vertical text on left margin reading 'Medicine name active ingredient 50 mg'
- Bold warning text 'PRESCRIPTION ONLY MEDICINE'
- Safety warning 'KEEP OUT OF REACH OF CHILDREN'
- Product name 'Medicine name' with '50 mg' dosage information
- Package quantity '10 tablets'
- Australian registration number 'AUST R XXXXX'
- Right side features include a green circular checkmark symbol, blue '50 mg' label, decorative
- blue balloon-like icon, and 'Sponsor' text.
- Bottom panel: Repeats 'Medicine name' and 'active ingredient 50 mg'.
The layout maintains consistent typography and spacing throughout, with the green checkmark symbol indicating an approved design. All text elements follow pharmaceutical labelling standards with clear hierarchy and readability.
Cohesive unit of medicine name and active ingredient name
Requirements
The name of the medicine and the name of active ingredient(s) on the main label must appear as a ‘cohesive unit’ (Subsection 9(3) of TGO 91).
The name and quantity of an active ingredient must be together and each active ingredient must be on a separate line of text. Active ingredient information must be immediately under the name of the medicine, without being separated by any text or graphics (except in certain circumstances).
Further details are outlined in Guidance on TGO 91 and TGO 92 (see Section 1.7.1).
Question
What is wrong with the following example?

Answer
The logo of the sponsor and ‘Each tablet contains’ interrupt the cohesive unit (highlighted in red dashed box).
Solution
Unclutter your cohesive unit. Move logo, strength and ‘each tablet contains’ out of the cohesive unit.
You can place certain information on the same line as the cohesive unit as long as it is spaced far enough away. For example, justified to the right when the cohesive unit is justified to the left. In this example, the ‘50 mg’ blue square graphic should be moved to the right of the label.
Useful tip
Only certain information should be present in the cohesive unit. All other text and graphics need to be moved or removed.

A pharmaceutical label design showing approved layout with cohesive unit annotation:
Header contains warning text: 'PRESCRIPTION ONLY MEDICINE' 'KEEP OUT OF REACH OF CHILDREN'
Central area features a dotted-line box with arrow pointing from 'Cohesive unit' label, containing:
'Medicine name' in large text 'active ingredient 50 mg'.
Below it Additional information includes:
- Package quantity '10 tablets' Registration number 'AUST R XXXXX'
- Right side elements:
- Large green checkmark symbol in a circle
- Blue pill-shaped label showing '50 mg'
- Decorative blue balloon-like icon
- Text 'Sponsor'.
- Red dotted lines and annotations demonstrate the cohesive unit relationship between elements.
The green checkmark indicates this is an approved design layout. All elements maintain clear visual hierarchy and proper spacing for readability.
Medicine name on three non-opposing sides
Requirements
Where the medicine is packaged in a primary package that is a carton, the name of the medicine must appear on at least 3 non-opposing sides of the carton (Paragraph 8(1)(o) of TGO 91). This allows pharmacists to identify medicines easily regardless of how they are stacked on shelves.
Further details are outlined in Guidance on TGO 91 and TGO 92.
Question
What is wrong with the following example?

A medical package label template showing a bad prescription medicine box layout. The template displays 'Medicine name' with '50 mg active ingredient' appearing on 2 sides.
Key elements include: a barcode, 'PRESCRIPTION ONLY MEDICINE' warning, 'KEEP OUT OF REACH OF CHILDREN' notice, package contents showing '10 tablets', an Australian registration number (AUST R XXXXX), spaces for batch number and expiry date, storage conditions noting 'Contains sugars as lactose', and a sponsor logo represented by a light blue balloon-like symbol. A red note indicates 'Medicine name is only on 2 sides'.
Answer
The name of the medicine is displayed only on the front and back panels (that is, on 2 opposing sides).
Solution
Include the name of the medicine on additional non-opposing carton panels.
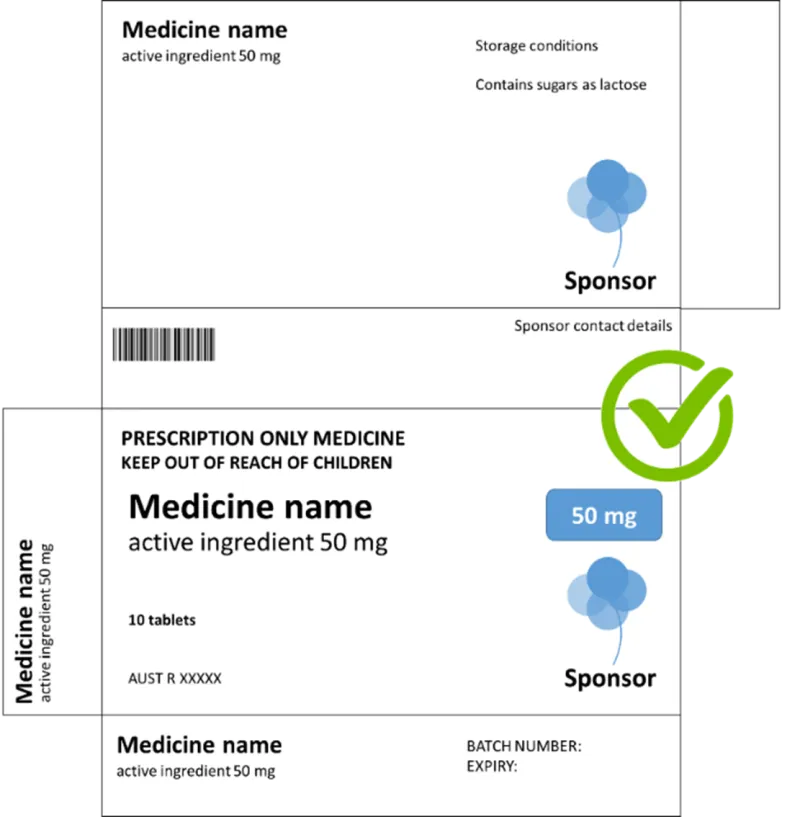
A template for prescription medicine packaging showing multiple panels of a medicine box layout that meets requirements. "medicine name" always appears with "active ingredient 50mg" directly underneath. This pairing of name and dosage is what makes this box correct.
The main panel features "PRESCRIPTION ONLY MEDICINE" and "KEEP OUT OF REACH OF CHILDREN" warnings prominently displayed, followed by "Medicine name" with "active ingredient 50 mg" underneath. The package contains 10 tablets and displays an AUST R number (XXXXX).
A blue balloon-like logo for "Sponsor" appears in multiple locations.
The top panel includes storage conditions and "Contains sugars as lactose" information.
A barcode is present on one panel, and there's a green checkmark icon in a circle. The bottom panel has spaces for batch number and expiry date.
The dosage "50 mg" is highlighted in a blue rectangular badge.
TGO 91 Schedule 1 substances
Requirements
Where a substance referred to in Schedule 1 of TGO 91 is present in the medicine, under the conditions outlined in Schedule 1, the name of the substance must be present on the labels. (Paragraph 8(1)(j) of TGO 91).
Schedule 1 is not limited to ingredients that are deliberately included in the medicine’s formulation. In some instances, you may need to assess your medicine for traces of substances that may be involved in the manufacturing process but are not actual ingredients, e.g. sulfur present in stabilising agents or milk products as growth media.
Further details are outlined in Guidance on TGO 91 and TGO 92 (see Section 1.5.9).
Question
What is wrong with the following example? For this example: the maximum daily dose of the product is 100 mg, which may be administered as two 50 mg tablets. Each tablet contains 60mg lactose.

A prescription medicine label with a white background and black text. At the top, there are two warning messages in capital letters: 'PRESCRIPTION ONLY MEDICINE' and 'KEEP OUT OF REACH OF CHILDREN'.
The main content shows 'Medicine name' with 'active ingredient 50 mg' below it. The package contains 10 tablets.
Below the words '10 tablets' are the words 'Contains lactose'. This co-location with the tablet number does not meet requirements.
An Australian registration number 'AUST R XXXXX' appears at the bottom. In the top right corner, there's a red circular prohibition symbol (X in a circle), and below it, a blue '50 mg' indicator and a light blue abstract flower or bubble design.
The word 'Sponsor' appears at the bottom right beside the blue design.
Answers
‘Sugars’ is not declared on the label.
Lactose is listed in Schedule 1of TGO 91. In this example, the dosage form is for oral administration therefore column 3 is applicable and ‘lactose’ must be declared on the label (column 4).
Also, lactose is a sugar that has a glycaemic effect so the entry ‘sugars – monosaccharides and disaccharides’ in Schedule 1 also applies. Because the maximum recommended daily dose of the medicine will contain more than 100 mg of lactose (that is, 2 × 50 mg tablets = 2 × 60 mg lactose per tablet = 120 mg lactose in maximum daily dose), column 2 and 3 are satisfied and ‘sugars’ must also be declared on the label.
Solution
Declare on the label that the medicine ‘Contains sugars as lactose.’
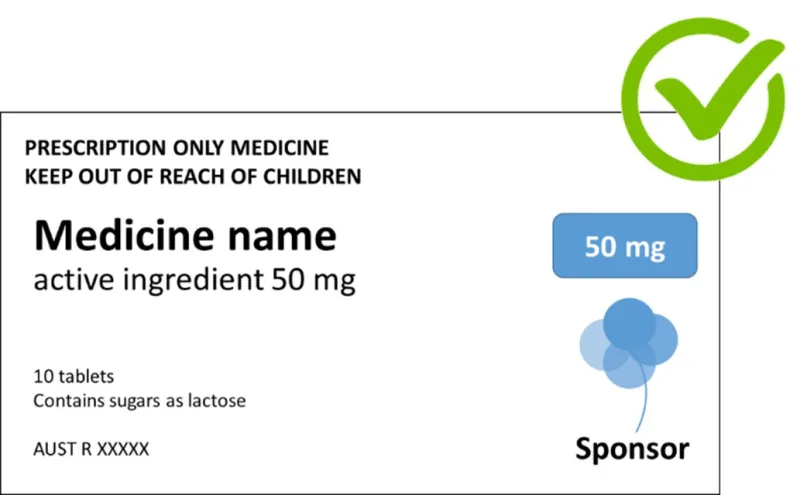
A prescription medicine label with a white background and black text. At the top, there are 2 warning messages in capital letters: 'PRESCRIPTION ONLY MEDICINE' and 'KEEP OUT OF REACH OF CHILDREN'.
The main content shows 'Medicine name' with 'active ingredient 50 mg' below it. Below the words '10 tablets' is a notice stating 'Contains sugars as lactose'.
This is the right way to label lactose. An Australian registration number 'AUST R XXXXX' appears at the bottom. In the top right corner, there's a green circular checkmark symbol, and below it, a blue '50 mg' indicator and a light blue abstract flower or bubble design.
The word 'Sponsor' appears at the bottom right beside the blue design.
Useful tips
You should check all excipients in the formulation to determine if multiple substances need to be declared. This includes checking components of proprietary ingredient mixtures (such as flavours or colours).
You do not need to include Schedule 1 substances on the main label, but they must be present somewhere on the labels.
Location of batch and expiry details
Requirements
TGO 91 requires that labels include both the:
- batch number of the medicine, preceded by the batch number prefix, and
- expiry date of the medicine, preceded by the expiry date prefix.
This information is required for all dosage forms on the container (for example, on a blister pack or vial label) and, if present, any other packaging (for example, the outer carton).
Options for how to present the batch and expiry prefixes are defined in Section 6 of TGO 91.
The batch number and expiry date must be preceded by the relevant prefixes, that is, the prefixes must be above or to the left of the related information.
Further details are outlined in Guidance on TGO 91 and TGO 92 (see Section 1.5.5).
Question
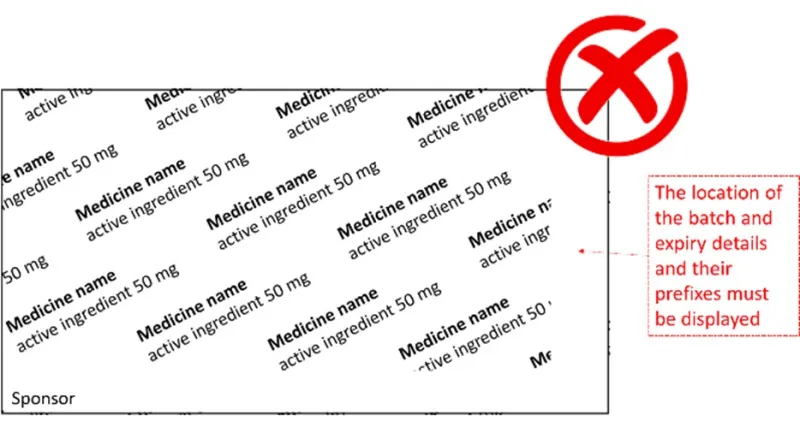
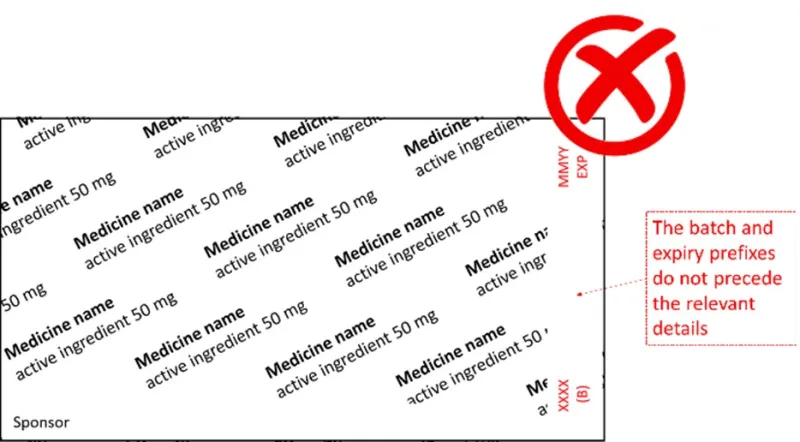
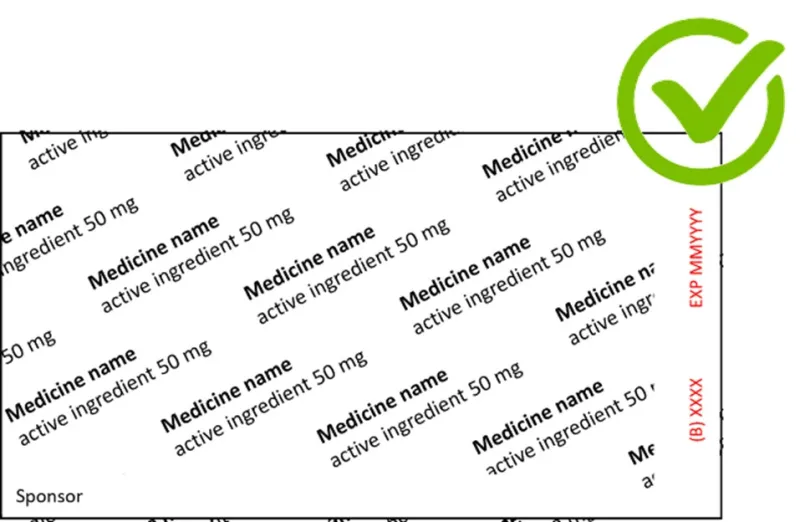
What is wrong with the following blister foil examples?
Answers
- In the first example, the location of the batch and expiry details and their prefixes are not shown.
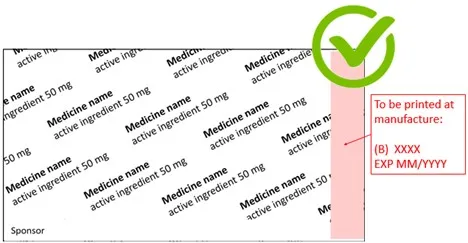
- In the second example, the batch and expiry prefixes do not precede the batch and expiry details.
Solution
Show how and where the prefixes and details will appear on the printed labels. Make sure that the batch and expiry prefixes precede (i.e. are to the left or immediately above) the batch and expiry details.
Useful tip
You can annotate the blister by:
- including wording of batch and expiry details, or
- identifying where the details will be located (highlighted in pink in the second example)

