Disclaimer: This guidance document was developed in 2017 and reflects the research on medicinal cannabis available up to that time. Clinical guidance is not usually the role of the TGA as a therapeutic goods regulator, however, the information was developed to support medical practitioners at the time. The TGA is discussing potential avenues for updating this research in the future.
Version history
- The clinical guidance documents were developed in 2017 and provide a broad overview of the evidence available at that time to support medicinal cannabis use.
- In February 2020, the National Drug and Alcohol Research Centre reviewed the clinical evidence for the use of medicinal cannabis published in refereed medical journals since the clinical guidance documents were released. Additional relevant studies were included in the bibliographies for epilepsy and pain.
- In November 2024, the clinical guidance documents were updated to reflect current regulatory information.
Important considerations
- Guidance documents are not the same as clinical guidelines. Guidance documents are documents produced to provide advice and further explanations and do not specify requirements that are binding in regulation.
- Healthcare practitioners are encouraged to conduct their own research to ensure their knowledge and prescribing reflects the current state of evidence of medicinal cannabis.
Further information
Visit the TGA’s medicinal cannabis consumer hub for additional information on how the TGA provides legal access to medicinal cannabis products in appropriate circumstances.
Introduction
In 2017, a set of guidance documents were made available to assist doctors and their patients who choose to prescribe medicinal cannabis in Australia under current access schemes. These were developed based on reviews of available evidence for the use of medicinal cannabis in five different settings at the time. Included is an overview addressing the evidence base for medicinal cannabis therapy generally as well as specific documents relating to medicinal cannabis in the treatment of palliative care, epilepsy, chemotherapy-induced nausea and vomiting (CINV), multiple sclerosis (MS) and chronic pain.
This document reflects the evidence supporting the use of medicinal cannabis in treating symptoms of MS, including pain, spasticity, bladder spasm, ataxia and tremor, adverse events, quality of life and disability and the recommendations of the Multiple Sclerosis Working Group.
Note: These guidance documents are based on evidence available at the time of publication (2017). Each document should be read in conjunction with the 'Guidance to the use of medicinal cannabis in Australia - Overview'.
Review method
In 2017, the Australian Government Department of Health commissioned a team from the University of New South Wales, University of Sydney and University of Queensland under the coordination of the National Drug and Alcohol Council (NDARC) to review the available evidence for the use of medicinal cannabis in the above five settings.
The researchers conducted a review of previously published reviews from multiple databases such as Medline, Embase, PsychINFO and EBM Reviews based on PRISMA145. PRISMA is the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and is an evidence-based minimum set of items for reporting on randomised controlled trials (RCTs). The guidelines were developed because of concern for low quality trials and they aim to improve the quality of medical research, remove bias and improve transparency and accurate reporting of findings. Searches were guided by a specialist Librarian using specific search terms and were limited to studies published between 1980 and early 2017. Two reviewers independently examined titles and abstracts for relevance using Covidence Software and the Cochrane Risk of Bias Tool was used to assess studies, aiming to increase accuracy. The GRADE (grading of recommendations, assessment, development and evaluation) approach, an internationally recognised standard applying to weighting of evidence in scientific and medical literature was used to evaluate the quality of evidence.
In July 2017, the Department also convened five separate Working Groups to consider the available evidence for the use of medicinal cannabis in the treatment of each of the settings. The five groups consisted of individuals from a wide range of backgrounds and organisations, including senior staff from each state and territory Department of Health, fifteen healthcare professional organisations, clinical staff from twenty-nine hospitals and healthcare systems, fourteen outpatient or Primary Health Networks and eighteen consumer representative groups.
Caveats
It should be noted that there are significant limitations in our knowledge of the medicinal use of cannabis and that the evidence/data is only current up to 2017.
This document provides guidance for health professionals in the use of an unapproved medicine, in the context of limited evidence of efficacy in the treatment of MS symptoms. There are few long-term studies and, other than for nabiximols (Sativex), there are limited data to advise on dose, tolerance and safety in people with MS.
This document includes dosing suggestions for cannabinoids including delta-9 tetrahydrocannabinol (THC) and cannabidiol (CBD), their combinations and routes of administration.
Evidence of benefit from medicinal cannabis use is limited.
- Guidance can only relate recommendations to the condition, drug and dose which have been studied. For example, evidence of efficacy in anorexia from one product and dose should not be extrapolated to pain control with the same product and dose.
- There are limitations in how the evidence was obtained and reviewed.
- Dose-response information is lacking, in particular for starting doses. This is particularly relevant when applying data from younger people to the elderly or people with cachexia, cognitive impairment and hepatic or renal disease.
- Dose-response information for toxicity is also lacking, particularly for side effects which may overlap with distress symptoms and may occur at different doses and before efficacy is evident. Side effects which are reversible in younger people when ceasing the cannabinoid product may be irreversible in this setting.
- There is no dose equivalence safety or efficacy data between products or between specific cannabinoids and current standard of care therapy.
As with all therapies, medical practitioners must exercise their professional judgment in determining whether medicinal cannabis products are an appropriate treatment for an individual patient. At this time, the use of medicinal cannabis should be considered only where conventional treatments have been proven unsuccessful in managing the patient's symptoms.
Summary of the current evidence
This document reviews the role of cannabinoids in treating the symptoms associated with multiple sclerosis, including:
- disability and disability progression;
- pain;
- spasticity;
- bladder function;
- ataxia and tremor;
- sleep; and
- quality of life.
A literature search for high quality systematic reviews was conducted on the use of cannabinoids to treat the symptoms of multiple sclerosis, with a cut-off date of November 30, 2016. A systematic review-of-reviews was conducted of studies that provided evidence on the use of cannabinoids as anti-emetics.
Overall, there is low to moderate quality evidence which suggests pharmaceutical-grade THC (dronabinol or THC extract) is effective for treating symptoms of pain.
THC:CBD (nabiximols, Sativex) may be effective for treating symptoms of pain and spasticity in MS, in certain patient populations.
Findings were mixed as to whether cannabinoids assisted in improving bladder function, sleep, patient quality of life, ataxia/tremor and disability/disease progression.
No studies included active alternatives (non-cannabinoid medicines) as comparators, which is an important limitation.
These results are based on 11 systematic reviews, which included 32 individual studies (see Table 1).
Key to grades - adapted from the Mayo Clinic[1]
A Strong scientific evidence for this use
B Good scientific evidence for this use
C Unclear scientific evidence for this use
D Fair scientific evidence against this use (it may not work)
F Strong scientific evidence against this use (it likely does not work)
Disability and disease progression
Six reviews, with a total of 11 individual studies, reported data on measures of patient disability or disease progression[2,3,4,5,6]. Few studies evaluated the effect of cannabis sativa and nabiximols in slowing disability and disease progression. Where there was evidence it suggested that they had no effect. Likewise, findings were inconsistent for the use of dronabinol and THC:CBD extracts as therapies to slow disability or disease progression. The overall lack of evidence for an effect of cannabinoids on disease progression was emphasised by the Working Group.
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| C | Cannabis sativa | One review included one study (one Randomised Control Trial - RCT) that reported evidence of low quality that cannabis sativa produced no change in patient disability or disease progression. |
| C | Dronabinol | Four reviews included four studies (two RCT) that provided very low to high quality evidence that reported inconsistent effects of dronabinol on disability and disease progression. |
| C | Nabiximols | Three reviews included two studies (two RCT) of moderate quality that reported nabiximols produced no change for patient disability or disease progression. |
| C | THC:CBD extracts | Six reviews included six studies (five RCT) of low to moderate quality that reported inconsistent effects of THC:CBD extracts on patient disability and disease progression. |
Pain
Seven reviews, including 19 individual studies, reported data on measures of pain[8,9,10,11,12,13,14]. There was some evidence that THC, including dronabinol and THC extracts, was effective in reducing pain. Findings were more inconsistent for nabiximols and THC:CBD extract combinations, with some reports of positive outcomes for pain. Two reviews concluded that cannabinoids were probably effective for the treatment of painful spasticity[15,16].
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| C | Cannabis sativa | One review included one study (one RCT) of low quality that reported cannabis sativa had a positive effect on pain. |
| B | Dronabinol | Six reviews included four studies (three RCT) of low to high quality that reported dronabinol had a positive effect in reducing pain. |
| B | THC extract | Three reviews included three studies (two RCT) of very low to low quality that reported THC extracts had a positive effect in reducing patient pain. |
| C | Nabiximols | Four reviews included eight studies (five RCT) of very low to moderate quality that reported inconsistent results of the effect of nabiximols in reducing patient pain. |
| C | THC:CBD extracts | Six reviews included seven studies (five RCT) of very low to high quality that reported inconsistent results of the effect of THC:CBD extracts in reducing pain. |
| C | Nabilone | Two reviews included one RCT of very low quality that reported a positive effect of nabilone in reducing pain. |
| C | CBD | Three reviews included two studies (2 RCT) of low quality that reported mixed results for the effectiveness of CBD in reducing pain. |
Spasticity
Early data in animal models of MS suggested improvement in spasticity from cannabinoids in humans.
Seven reviews, with a total of 20 individual studies, reported data on changes in patient measures of spasticity[17,18,19,20,21,22,23]. Findings were inconsistent for the use of nabiximols and THC:CBD extract combinations. There was some evidence from moderate quality studies that nabiximols reduced spasticity as reported by patient ratings. A number of reviews concluded that cannabinoids (particularly THC:CBD combinations) were probably effective in reducing spasticity[24,25,26].
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| C | Cannabis sativa | Two reviews included two studies (two RCT) of low quality that reported cannabis sativa had reduced patient spasticity. |
| C | Dronabinol | Six reviews included five studies (five RCT) of low to high quality reported mixed findings on the effectiveness of dronabinol in reducing patient spasticity. |
| C | THC extract | Four reviews included two studies (one RCT) of very low to low quality that reported THC extracts reduced patient spasticity. |
| C | Nabiximols | Five reviews included seven studies (six RCT) of very low to moderate quality that reported inconsistent findings on the effectiveness of nabiximols in reducing patient spasticity. |
| C | THC:CBD extracts | Seven reviews included six studies (five RCT) of low to high quality that reported inconsistent findings on the effectiveness of THC:CBD extracts in reducing patient spasticity. |
| C | Nabilone | One review included two studies (two RCT) of very low to low quality that reported nabilone had a positive effect on spasticity. |
| C | CBD | One review included one low quality RCT that reported CBD likely did not have an effect on patient spasticity. |
Bladder function
Four reviews, with a total of seven individual studies, reported the effects of cannabinoids on patient bladder function [27,28,29,30]. Evidence across all cannabinoids tested was insufficient or inconsistent. Two reviews concluded that nabiximols and THC extract were effective at reducing urinary incontinence or the number of bladder voids per day but these conclusions were based on a single study [31,32].
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| C | Dronabinol | Two reviews included two studies (one RCT) of high quality that reported mixed results for dronabinol in improving bladder function. |
| C | THC extract | One review included one very low quality study (zero RCT) that reported THC had a positive effect in improving patient bladder function. |
| C | Nabiximols | Two reviews included two studies (two RCT) of moderate quality that reported nabiximols mixed effects on patient bladder functioning. |
| C | THC:CBD extracts | Two reviews included four studies (two RCT) of very low to high quality reported mixed findings on the effect of THC:CBD extracts on bladder functioning. High quality studies reported that there was no significant improvement in patients receiving THC:CBD. |
| C | Nabilone | Two reviews included one low quality RCT that reported nabilone had no effect on patient bladder functioning. |
Ataxia and tremor
Four reviews, with a total of eight individual studies, reported changes to patient ataxia and tremor [33,34,35,36]. Evidence was based on small studies, and most cannabinoids had no significant effect of ataxia and tremor. Two reviews concluded that cannabinoids were probably ineffective or produced no significant benefit in treating patient tremor [37,38].
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| D | Dronabinol | Three reviews included three studies (two RCT) of very low to high quality that reported dronabinol had mixed effects on patient ataxia and tremor. |
| D | Nabiximols | Three reviews included two studies (two RCT) of moderate quality reported that nabiximols had no effect on patient ataxia or tremor. |
| D | THC:CBD extracts | Three reviews included four studies (four RCT) of low to high quality reported mixed results of the effect of THC:CBD extracts on ataxia and tremor. The high quality RCT reported no significant changes to patient tremor in those receiving THC:CBD extracts. |
| D | Nabilone | One review included one low quality RCT that reported there was no effect of nabilone in reducing patient ataxia and tremor. |
Sleep
Three reviews, with a total of six individual studies, reported effects of cannabinoids on patient sleep quality[39,40,41,42]. There was evidence from one study that nabiximols were effective at improving sleep quality. One review noted the studies included indicated a positive effect of cannabinoids on sleep quality [43].
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| C | Dronabinol | Three reviews included two studies (one RCT) of moderate to high quality that reported mixed results, mostly indicating a positive effect on sleep. |
| C | THC extract | One review included three studies (two RCT) of very low to low quality that reported mixed effects of THC extracts on patient sleep and sleep quality. |
| C | Nabiximols | One review included one moderate quality RCT that reported nabiximols had a positive effect on patient sleep quality. |
| C | THC:CBD extracts | Three reviews included four studies (three RCTs) of low to high quality that reported mixed (mostly positive) effects of THC:CBD extracts on patient sleep quality. |
| C | CBD extract | One review included one low quality RCT that reported CBD had a positive effect on patient sleep quality. |
Quality of life
Four reviews, with a total of 12 individual studies, reported on the effects of cannabinoids on patient quality of life [44,45,46,47]. Findings were inconsistent across the cannabinoids. There was some moderate quality evidence that nabiximols were more effective than placebo at improving patient global impression of change. One meta-analysis reported the mean number of patients reporting improved global impression of change scores was greater for nabiximols than placebo [48]. Studies of other cannabinoids gave little or no evidence that they improved patient quality of life.
| Evidence Grade | Cannabinoid used | Outcomes |
|---|---|---|
| C | Cannabis sativa | Two reviews included two low quality RCTs that reported some patients experienced improvement in overall quality of life, however clinical measures were not significant. |
| C | Dronabinol | Three reviews included two low to high quality RCTs that reported mixed findings. The high-quality study reported that there was no significant change in patient general health scores. |
| B | Nabiximols | One review included five RCTs of moderate quality that reported inconsistent results. The average number of patients who reported an improvement on global impression of change was greater for nabiximols than placebo. |
| C | THC:CBD extracts | Four reviews included three RCTs of low to high quality that reported mixed results. The high-quality study reported no significant difference between cannabinoids and placebo for measures of patient quality of life. |
| C | Nabilone | Two reviews included two RCTs of very low to moderate quality that reported mixed results. There was some evidence that nabilone improved patient global impression, however sample sizes were very small. |
Recommendation
There is some evidence that dronabinol or THC extracts may be effective at reducing pain associated with multiple sclerosis. There is also some evidence (although inconsistent) that nabiximols and other THC:CBD extracts may reduce muscle spasticity and improve patient quality of life.
Recommendations are limited by lack of quality evidence. Currently available studies demonstrate no evidence of an effect of cannabinoids on MS disease activity or disability progression. There have been no studies comparing cannabinoids against current standard treatments for multiple sclerosis.
Adverse effects
Commonly reported adverse events in trials in MS included dizziness, somnolence dysphoria, euphoria, feeling 'high', diarrhoea, and vertigo. Most reviews classified these adverse events as mild or well tolerated.
Acute administration of cannabis to elderly or particularly sensitive patients should be considered carefully, and psychotic or 'particularly vulnerable' patients should avoid the chronic use of cannabinoids[49]. Koppel et al [50] also noted that cognitive impairment is likely to be of concern. Some patients who have neurologic conditions may have pre-existing cognitive dysfunction, which may increase their susceptibility to cannabinoids' toxicities.
Combined extracts of THC and CBD may attenuate side effects associated with THC alone[51]. The incidence of side effects varies greatly and depends on the amount of cannabis needed to limit spasticity.
In a meta-analysis of adverse events associated with medical cannabinoid use, Wang et al[52]. reported that the most frequently reported adverse events were nervous system disorders. Serious adverse events included 21 instances of relapse of multiple sclerosis, serious convulsion, and severe dizziness.
Recent reviews have suggested as many as 10 per cent of adults who use cannabis develop psychological dependence and that percentage may be higher in younger age groups[53]. There is no evidence to provide guidance on drug-drug interactions. If cannabinoids are to be used in conjunction with other therapies, clinicians and patients should be aware of common adverse events associated with cannabinoid use and consider whether these events are likely to interfere with quality of life.
Patients and prescribing clinicians should be aware of likely adverse events such as dizziness, somnolence, dysphoria and diarrhoea. Clinicians considering cannabinoid therapy for patients should consider the individual's capacity for using cannabinoids for long periods of time.
Place in therapeutic hierarchy
It is difficult to evaluate where cannabinoids could usefully be placed in the therapeutic hierarchy because all trials have compared cannabinoids to placebo rather than other therapies. Several reviews concluded that cannabinoids may be effective or beneficial for the treatment of spasticity or pain associated with multiple sclerosis but made no recommendations about their place in the therapeutic hierarchy [54,55,56,57,58].
To determine the relative efficacy of cannabinoids as treatments for spasticity or pain, trials would need to compare cannabinoids to standard first and second-line treatments used to treat multiple sclerosis.
Recommendation
In the absence of evidence comparing cannabinoids to first line treatments for pain and spasticity in MS, including baclofen, dantrolene, and benzodiazepines, there is no basis for using cannabinoids as a monotherapy or first line treatment. If pain and spasticity are not properly controlled by standard therapies, doctors may discuss with their patients the use of nabiximols or dronabinol as an adjunctive therapy.
Evidence on time to response
Treatment duration in randomised controlled trials and open label clinical trials was a median of four weeks (range one day to 52 weeks). Three studies evaluated cannabinoids for up to two years [59,60,61]. A number of studies had maintenance phases for patients after titrating to their effective cannabinoid dose [62,63,64,65,66]. None of the reviews made statements about typical time to response.
Recommendation
In the absence of strong evidence for dosing and particular preparations of cannabis or cannabinoids in the treatment of symptoms of multiple sclerosis (other than nabixomols), it is recommended that any treating physician who elects to initiate cannabinoid therapy should re-evaluate patients after four to six weeks for evidence of response to treatment.
Use of THC/CBD combinations or products
The majority of studies (21) evaluating the use of cannabinoids in treating symptoms of multiple sclerosis used THC/CBD combinations.
Nabiximols (THC:CBD), trade name Sativex, were most commonly tested. There was some evidence that they may be effective for reducing patient pain and spasticity and may improve sleep and quality of life. THC:CBD or nabiximols were the only cannabinoid products that studies assessed all the identified outcomes used to evaluate effectiveness.
Dosage forms, variations in route of administration and standardisation
| Cannabinoid product | Preparation | Administration | Standardised |
|---|---|---|---|
| Nabiximols | Liquid | Oromucosal spray | Yes |
| THC:CBD extracts | Liquid | Sublingual spray | Yes |
| Capsule | Oral | Yes | |
| Dronabinol | Capsule | Oral | Yes |
| THC extract | Liquid | Spray | Yes |
| Nabilone | Capsule | Oral | Yes |
| CBD | Liquid | Spray | Yes |
| Cannabis sativa | Cigarette | Smoked | Not specified |
Nabiximols
Nabiximols were administered as a standardised oromucosal liquid spray. Studies using nabiximols addressed all eight outcomes identified as indicators of effectiveness and safety for treatment for symptoms of multiple sclerosis[67,68,69,70,71,72,73,74,75,76,77]..
THC:CBD extracts
THC:CBD extracts were administered as either a standardised oromucosal liquid spray or an oral capsule. Studies using THC:CBD extracts addressed all eight outcomes identified as indicators of effectiveness and safety for treatment of symptoms of multiple sclerosis[78,79,80,81,82,83,84,85,86,87].
Dronabinol
Dronabinol was administered in a standardised oral capsule form. Studies using dronabinol addressed all eight outcomes identified as indicators of effectiveness and safety for treatment of symptoms of multiple sclerosis[88,89,90,91,92,93,94,95,96].
THC extracts
THC extracts were administered in standardised liquid form either as an oromucosal or sublingual spray. Studies using THC extracts did not address disability/disease progression and quality of life outcomes as indicators of effectiveness and safety for the treatment of symptoms of multiple sclerosis[97,98,99].
Nabilone
Nabilone was administered in a standardised oral capsule form. Studies using nabilone did not address disability/disease progression and change to sleep outcomes as indicators of effectiveness and safety for the treatment of symptoms of multiple sclerosis[100,101,102,103].
CBD extracts
CBD extracts were administered as a standardised liquid sublingual spray. Studies using CBD extracts addressed four of the eight identified outcomes as indicators of treatment effectiveness and safety, namely changes to pain, spasticity, sleep, and adverse events[104,105].
Cannabis sativa
Cannabis sativa was administered in a herbal cigarette and was unlikely to be a standardised product. Studies using cannabis sativa addressed five of the eight identified outcomes as indicators of treatment effectiveness and safety, namely change to disability/disease progression, pain, spasticity, quality of life, and adverse events[106,107].
Recommendation
For patients who may benefit from the use of cannabinoids in treating pain or spasticity from multiple sclerosis, it is recommended that a physician who elects to initiate cannabinoid therapy use standardised products, and pharmaceutical-grade nabiximols, dronabinol, or THC extract produced with GMP (good manufacturing practice) which have the greatest evidence for efficacy based on the review.
Dose (including various cannabinoids in the product), dose ranges for which there is evidence, other pharmacological considerations for dosages
Nabiximols
Studies reported patients receiving nabiximols received the standardised oromucosal spray which delivers 2.7mg THC and 2.5mg CBD per spray. Patients were able to administer between 12 and 48 sprays per 24 hours. In studies where there was evidence of effectiveness, doses ranged between 12 and 48 sprays per day[108,109,110,111,112,113,114,115].
The Mayo Clinic reports that, to treat symptoms of multiple sclerosis, 2.5–120 mg in divided doses (eight sprays within three hours, up to 48 sprays in 24 hours) has been used for 6 to 14 weeks[116].
THC:CBD extracts
Studies reported patients receiving THC:CBD extracts received either capsule or sublingual sprays. Dosages for capsules ranged from 2.5mg and up to 12.5mg of THC, and 0.8mg and up to 2.5mg of CBD. Capsules were given two to four times per day. Dosages for sublingual sprays administered 2.5mg THC and 2.5mg CBD, up to 48 times per day. In studies where there was evidence for effectiveness, capsule doses ranged between 2.5mg and 12.5mg of THC, and 0.8mg to 1.8mg CBD, administered two to four times per day[117,118,119,120]. Effective sublingual sprays administered 2.5mg THC and 2.5mg CBD up to 48 times per day[121,122,123].
The Mayo clinic reports that, to treat symptoms of multiple sclerosis, cannabis extracts with THC:CBD combinations ranging between 2.5–120mg has been taken by mouth daily for two to 15 weeks[124].
Dronabinol
Studies reported patients receiving dronabinol in standardised capsule form. Dosage ranged from 2.5–15mg, received between one and four times per day. Where there is evidence for effectiveness, dose ranges were between 2.5mg and 15mg, administered between one and four times per day[125,126,127,128,129,130].
The Mayo Clinic reports that to treat multiple sclerosis symptoms, 2.5mg of dronabinol is taken by mouth daily, increasing to a maximum of 10mg daily for three weeks[131].
THC extract
One study reported patients received 2.5mg of THC as a sublingual spray, up to 48 times per day. This dose was reported to be effective[132].
Nabilone
Studies reported patients received nabilone as a standardised capsule. Dosage ranged from 0.5mg–1.0mg, and in one study 0.03mg/kg. Dosage ranged between one and two capsules a day, and in one study, was administered every second day. Where there was evidence of effectiveness, dosages ranged between 0.5mg–1.0mg, and were administered one to two times per day, or in one case study, every second day[133,134,135].
CBD extract
Two studies reported that patients received CBD extract as a sublingual spray. They received 2.5mg of CBD per spray and were able to administer up to 48 sprays per day. There was evidence that this dosage range and schedule were effective[136,137].
Cannabis sativa
Two studies reported the use of cannabis sativa as a herbal cigarette. Dosages could not be accurately reported, but THC content ranged between 1.54 per cent and four per cent. Where there was evidence for effectiveness, one study reported that patients smoked one cigarette with four per cent THC content[138].
The Mayo Clinic reports cannabis extract capsules of 15–30mg have been taken by mouth, in 5mg increments, based on tolerance, for 14 days. Cannabis extracts such as Cannador have been taken by mouth for two to four weeks[139].
Tolerance and persistence in treatment
In the studies included in the review, treatment with cannabinoids appeared to be well tolerated but patients receiving them were more likely to withdraw from trials for any reason and due to adverse events. In a systematic review and meta-analysis of the use of cannabinoids to treat neurological disorders, 6.9 per cent of patients receiving cannabinoids stopped treatment because of adverse events compared to 2.2 per cent of patients who received placebo[140]. In longer-term treatment, two open-label extension studies were associated with withdrawal rates of up to 25 per cent[141]. Comparison to standard treatments for pain and spasticity in multiple sclerosis is needed to determine whether patients are significantly more likely to withdraw from cannabinoids than other multiple sclerosis treatments.
Recommendation
If treatment is likely to be long term, it is important that any side-effects from cannabinoids are not greater than the side effects experienced with other medications. This requires their response to treatment to be regularly assessed. Measures of tolerability include experience of adverse event and patient assessment of treatment efficacy.
Stopping rules
There is no current high quality evidence in multiple sclerosis symptom clusters.
There is little information on dose-response. Starting doses should be low, and the dose increased in response to lack of efficacy until toxicity outweighs any benefit.
In the absence of strong evidence for dosing and specific preparations of cannabis or cannabinoids in the treatment of multiple sclerosis symptoms it is recommended that any treating physician who elects to initiate cannabinoid therapy should re-evaluate the effectiveness and adverse effects of the cannabinoid medication after 12 weeks of therapy.
Information on pharmacovigilance should be collected by the prescribing doctor. This will help refine guidance documents and provide additional data.
NDARC Review
Figure 1. PRISMA Chart
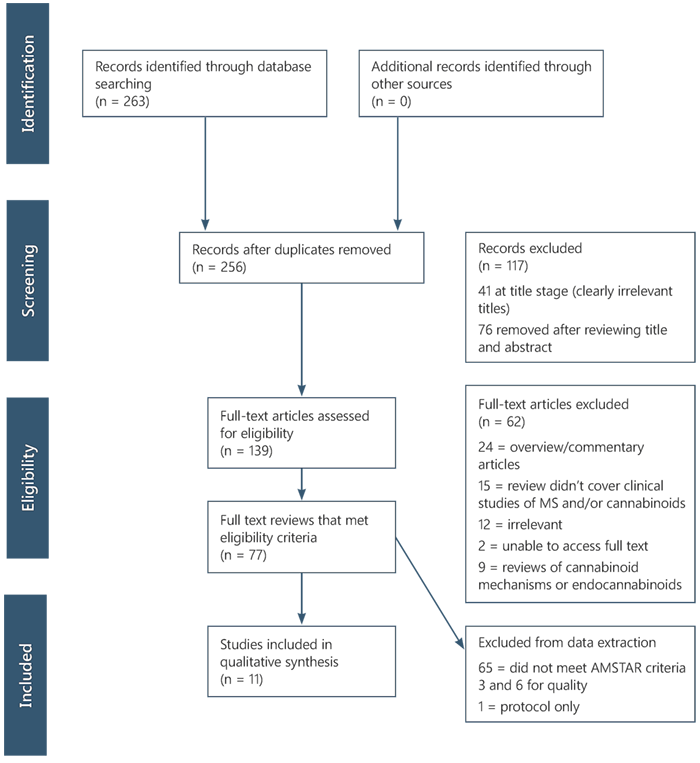
Text version of Figure 1
Identification
- Records identified through database searching (n = 263)
- Additional records identified through other sources (n = 0)
Screening
- Records after duplicates removed (n = 256)
Records excluded (n = 117)
41 at title stage (clearly irrelevant titles)
76 removed after reviewing title and abstract
Eligibility
- Full-text articles assessed for eligibility (n = 139)
Full-text articles excluded (n = 62)
24 = overview/commentary articles
15 = review didn't cover clinical studies of MS and/or cannabinoids
12 = irrelevant
2 = unable to access full text
9 = reviews of cannabinoid mechanisms or endocannabinoids
- Full text reviews that met eligibility criteria (n = 77)
Included
- Studies included in qualitative synthesis (n = 11)
Excluded from data extraction
65 = did not meet AMSTAR criteria 3 and 6 for quality
1 = protocol only
| Disability and disease progression | Pain | Spasticity | Bladder function | Ataxia and tremor | Sleep | Quality of life | Adverse events | |
|---|---|---|---|---|---|---|---|---|
| Cannabis sativa (smoked) | 1 study (1 RCT) | 1 study (1 RCT) | 2 studies (2 RCT) | No studies | No studies | No studies | 2 studies (2 RCT) | 2 studies (2 RCT) |
| Findings | No change | Positive effect | Positive effect | Mixed effect | AEs > comparator | |||
| Quality of evidence | Low quality | Low quality | Low quality | Low quality | Low quality | |||
| Conclusion | Insufficient evidence | Insufficient evidence | Insufficient evidence | Insufficient evidence | Insufficient evidence | |||
| Dronabinol | 4 studies (2 RCT) | 4 studies (3 RCT) | 5 studies (5 RCT) | 2 studies (1 RCT) | 3 studies (2 RCT) | 2 studies (1 RCT) | 2 studies (2 RCT) | 8 studies (6 RCT) |
| Findings | No change/ negative effect | Positive effect | Mixed effect | Mixed effect | No change | Mixed effect (mostly positive) | Mixed effect | AEs > comparator |
| Quality of evidence | Very low to high quality | Low to high quality | Low to high quality | High quality | Very low to high quality | Moderate to high quality | Low to high quality | Very low to high quality |
| Conclusion | Inconsistent evidence | Some evidence of positive effect | Inconsistent evidence | Inconsistent evidence | Unlikely to have an effect | Insufficient evidence | Insufficient evidence | Mild AEs likely |
| THC extract | No studies | 3 studies (2 RCT) | 2 studies (1 RCT) | 1 study (no RCT) | No studies | 3 studies (2 RCT) | No studies | 1 study (1 RCT) |
| Findings | Positive effect | Positive effect | Positive effect | Mixed effect | AEs > comparator | |||
| Quality of evidence | Very low to low quality | Very low to low quality | Very low quality | Very low to low quality | Low quality | |||
| Conclusion | Some evidence of effect | Insufficient evidence | Insufficient evidence | Insufficient evidence | Mild AEs likely | |||
| Nabiximols | 2 studies (2 RCT) | 8 studies (5 RCT) | 7 studies (6 RCT) | 2 studies (2 RCT) | 2 studies (2 RCT) | 1 study (1 RCT) | 5 studies (5 RCT) | 10 studies (7 RCT) |
| Findings | No change | Mixed effect | Mixed effect | Mixed effect | No change | Positive effect | Mixed findings | AEs > comparator |
| Quality of evidence | Moderate quality | Very low to moderate quality | Very low to moderate quality | Moderate quality | Moderate quality | Moderate quality | Moderate quality | Very low to moderate quality |
| Conclusion | Insufficient evidence | Inconsistent evidence | Inconsistent evidence | Insufficient evidence | Unlikely to have an effect | Insufficient evidence | Some evidence of positive effect | Mild AEs likely |
| THC:CBD extracts | 6 studies (5 RCT) | 7 studies (5 RCT) | 6 studies (5 RCT) | 4 studies (2 RCT) | 4 studies (4 RCT) | 4 studies (3 RCT) | 3 studies (3 RCT) | 8 studies (6 RCT) |
| Findings | Mixed effect | Mixed findings | Mixed findings | Mixed findings | No change | Mostly positive effect | Mixed findings | AEs > comparator |
| Quality of evidence | Low to high quality | Very low to high quality | Low to high quality | Very low to high quality | Low to high quality | Low to high quality | Low to high quality | Low to high quality |
| Conclusion | Inconsistent evidence | Inconsistent evidence | Inconsistent evidence | Inconsistent evidence | Unlikely to have an effect | Some evidence of effect | Inconsistent evidence | Mild AEs likely |
| Nabilone | No studies | 1 study (1 RCT) | 2 studies (2 RCT) | 1 study (1 RCT) | 1 study (1 RCT) | No studies | 2 studies (2 RCT) | 3 studies (3 RCT) |
| Findings | Positive effect | Positive effect | Positive effect | No change | Mixed effect | AEs > comparator | ||
| Quality of evidence | Very low quality | Very low to low quality | Low quality | Low quality | Very low to moderate quality | Very low to low quality | ||
| Conclusion | Insufficient evidence | Insufficient evidence | Insufficient evidence | Insufficient evidence | Insufficient evidence | Mild AEs likely | ||
| CBD extract | No studies | 2 studies (2 RCT) | 1 study (1 RCT) | No studies | No studies | 1 study (1 RCT) | No studies | 1 study (1 RCT) |
| Findings | Mixed effect | Mixed findings | Positive effect | AEs > comparator | ||||
| Quality of evidence | Low quality | Low quality | Low quality | Low quality | ||||
| Conclusion | Insufficient evidence | Insufficient evidence | Insufficient evidence | Insufficient evidence |
Appendix A
NDARC Review
This review is a comprehensive 'review of reviews'[142] of high quality systematic reviews assessing the effectiveness of cannabinoids in treating the symptoms of multiple sclerosis. The objectives are to identify the cannabinoids used, including plant and pharmaceutical formulations, and assess their ability to improve patient experiences of disability, pain and spasticity, as well as improved quality of life. The review also considers tolerability and safety data, as reported by patient study withdrawals and reported adverse events. Each included review had to address at least one of the outcomes defined on the basis of clinical experience, namely:
- Disability and disability progression
- Pain
- Spasticity
- Bladder function
- Ataxia and tremor
- Sleep
- Quality of life
- Adverse effects
Papers describing mechanisms of cannabinoid action, commentaries and clinical overviews that did not present the results of studies were not included in the review.
Review quality was assessed using the AMSTAR measurement tool of methodological quality of systematic reviews[143]. The AMSTAR tool documents assessed risk of bias at the review level. To identify reviews conducted methodologically, and to minimise bias at the review level in study selection, each identified review was required to meet criterion three and six of the AMSTAR tool at a minimum. This reflects reviews that were conducted with a comprehensive search, and those that, at a minimum, describe the characteristics of the included studies.
Each individual study included in the review was also graded according to the GRADE criteria[144]. RCTs were considered high quality evidence, but may be downgraded to moderate or low quality due to bias, sample size, or other issues around sample size. Observational studies were considered to be low to very low quality evidence, and case series or case studies were considered to be very low quality evidence.
Footnotes
- The Mayo Clinic. Marijuana (cannabis sativa) - dosing. Drugs and supplements 2013 Nov 1, 2013 [cited 2017 April 3]; Available from: http://www.mayoclinic.org/drugs-supplements/ marijuana/dosing/hrb-20059701.
- Ben Amar, M., Systematic review: A review of their therapeutic potential. Journal of Ethnopharmacology, 2006. 105: 1-25.
- Karst, M., S. Wippermann, and J. Ahrens, Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs, 2010. 70: 2409-38.
- Lakhan, S.E. and M. Rowland, Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: A systematic review. BMC Neurology Vol 9 2009, ArtID 59, 2009. 9.
- Shakespeare, D.T., M. Boggild, and C. Young, Anti-spasticity agents for multiple sclerosis. Cochrane Database of Systematic Reviews, 2003(4): CD001332.
- Whiting, P.F., et al., Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA, 2015. 313: 2456-73.
- Ibid.
- Ben Amar, Systematic review
- Karst et al, Role of cannabinoids
- Shakespeare et al, Anti-spasticity agents
- Whiting et al, Cannabinoids for Medical Use
- Zhornitsky, S. and S. Potvin, Cannabidiol in humans - The quest for therapeutic targets. Pharmaceuticals, 2012. 5: 529-552.
- Jawahar, R., et al., A systematic review of pharmacological pain management in multiple sclerosis. Drugs, 2013. 73: 1711-22.
- Koppel, B.S., et al., Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology, 2014. 82: 1556-63.
- Karst et al, Role of cannabinoids
- Koppel et al, Systematic review
- Ben Amar, Systematic review
- Karst et al, Role of cannabinoids
- Lakhan et al, Whole plant cannabis extracts in the treatment of spasticity
- Shakespeare et al, Anti-spasticity agents
- Whiting et al, Cannabinoids for Medical Use
- Zhornitsky, S., Cannabidiol in humans
- Koppel et al, Systematic review
- Lakhan et al, Whole plant cannabis extracts in the treatment of spasticity
- Whiting et al, Cannabinoids for Medical Use
- Koppel et al, Systematic review
- Ben Amar, Systematic review
- Karst et al, Role of cannabinoids
- Whiting et al, Cannabinoids for Medical Use
- Koppel et al, Systematic review
- Whiting et al, Cannabinoids for Medical Use
- Koppel et al, Systematic review
- Ben Amar, Systematic review
- Koppel et al, Systematic review
- Andrzejewski, K., R. Barbano, and J. Mink, Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia, 2016. 6: 173-181.
- Mills, R.J., L. Yap, and C.A. Young, Treatment for ataxia in multiple sclerosis. Cochrane Database of Systematic Reviews, 2009(4).
- Koppel et al, Systematic review
- Mills et al, Treatment for ataxia in multiple sclerosis
- Ben Amar, Systematic review
- Karst et al, Role of cannabinoids
- Whiting et al, Cannabinoids for Medical Use
- Mills et al, Treatment for ataxia in multiple sclerosis
- Zhornitsky, S., Cannabidiol in humans
- Ben Amar, Systematic review
- Karst et al, Role of cannabinoids
- Whiting et al, Cannabinoids for Medical Use
- Mills et al, Treatment for ataxia in multiple sclerosis
- Whiting et al, Cannabinoids for Medical Use
- Ben Amar, Systematic review
- Koppel et al, Systematic review
- Lakhan et al, Whole plant cannabis extracts in the treatment of spasticity
- Wang, T., et al., Adverse effects of medical cannabinoids: a systematic review. CMAJ Canadian Medical Association Journal, 2008. 178: 1669-78.
- Wilkinson ST, Yarnell S, Radhakrishran R, Ball SA, D'Souza DC. Marijuana legalization: impact on physicians and public health. Ann Rev Med 2016:67; 453-466
- Ben Amar, Systematic review
- Karst et al, Role of cannabinoids
- Lakhan et al, Whole plant cannabis extracts in the treatment of spasticity
- Whiting et al, Cannabinoids for Medical Use
- Koppel et al, Systematic review
- Rog, D.J., T.J. Nurmikko, and C.A. Young, Oromucosal δ9-tetrahydrocannabinol/ cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clinical therapeutics, 2007. 29: 2068-2079.
- Wade, D.T., et al., Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Multiple Sclerosis Journal, 2006. 12: 639-645.
- Zajicek, J., et al., Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. Journal of Neurology, Neurosurgery & Psychiatry, 2005. 76: 1664-1669.
- Leocani, L., et al., Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: a double-blind, placebocontrolled, crossover study. Neurol Sci, 2009. 30: 531‑4.
- Killestein, J., et al., Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. Journal of Neuroimmunology, 2003. 137: 140-143.
- Zajicek, J., et al., Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. The lancet, 2003. 362: 1517-1526.
- Zajicek, J.P., et al., Multiple sclerosis and extract of cannabis: results of the MUSEC trial. Journal of Neurology, Neurosurgery á Psychiatry, 2012. 83: 1125-1132.
- Turcotte, D., et al., Nabilone as an adjunctive to gabapentin for multiple sclerosis‐induced neuropathic pain: a randomized controlled trial. Pain Medicine, 2015. 16: 149-159.
- Centonze, D., et al., Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurological Sciences, 2009. 30: 531.
- Collin, C., et al., Randomized controlled trial of cannabis‐based medicine in spasticity caused by multiple sclerosis. European Journal of Neurology, 2007. 14: 290-296.
- Collin, C., et al., A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurological research, 2010. 32: 451-459.
- Conte, A., et al., Cannabinoid‐induced effects on the nociceptive system: A neurophysiological study in patients with secondary progressive multiple sclerosis. European Journal of Pain, 2009. 13: 472-477.
- Kavia, R., et al., Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Multiple Sclerosis Journal, 2010. 16: 1349-1359.
- Langford, R., et al., A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. Journal of neurology, 2013. 260: 984-997.
- Leocani, L., et al., Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: a double-blind, placebo-controlled, crossover study. Neurol Sci, 2009. 30: 531‑4.
- Rog, D.J., et al., Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology, 2005. 65: 812-819.
- Rog et al, Oromucosal δ9-tetrahydrocannabinol/cannabidiol
- Wade, D.T., et al., Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Multiple Sclerosis Journal, 2006. 12: 639-645.
- Wade, D.T., et al., Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Multiple Sclerosis Journal, 2004. 10: 434-441.
- Brady, C., et al., An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Multiple Sclerosis, 2004. 10: 425-433.
- Fox, P., et al., The effect of cannabis on tremor in patients with multiple sclerosis. Neurology, 2004. 62: 1105-1109.
- Freeman, R., et al., The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). International Urogynecology Journal, 2006. 17: 636-641.
- Killestein et al, Immunomodulatory effects
- Killestein, J., et al., Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology, 2002. 58: 1404-1407.
- Notcutt, W., et al., Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1'studies. Anaesthesia, 2004. 59: 440-452.
- Vaney, C., et al., Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebocontrolled, crossover study. Multiple Sclerosis, 2004. 10: 417-424.
- Wade, D.T., et al., A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clinical rehabilitation, 2003. 17: 21-29.
- Zajicek et al., Cannabinoids for treatment of spasticity
- Zajicek et al., Cannabinoids in multiple sclerosis (CAMS) study
- Zajicek, et al., Multiple sclerosis and extract of cannabis
- Killestein et al, Immunomodulatory effects
- Killestein, J., et al., Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology, 2002. 58: 1404-1407.
- Zajicek et al., Cannabinoids for treatment of spasticity
- Zajicek et al., Cannabinoids in multiple sclerosis (CAMS) study
- Clifford, D.B., Tetrahydrocannabinol for tremor in multiple sclerosis. Annals of Neurology, 1983. 13: 669-671.
- Petro, D.J. and C. Ellenberger, Treatment of Human Spasticity with Δ9‐Tetrahydrocannabinol. The Journal of Clinical Pharmacology, 1981. 21(S1).
- Svendsen, K.B., T.S. Jensen, and F.W. Bach, Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ, 2004. 329: 253.
- Ungerleider, J.T., et al., Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Advances in alcohol & substance abuse, 1988. 7: 39-50.
- Brady et al., An open-label pilot study of cannabis-based extracts
- Notcutt et al, Initial experiences with medicinal extracts of cannabis
- Wade et al, A preliminary controlled study
- Fox, S.H., et al., Randomised, double‐blind, placebo‐controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Movement Disorders, 2002. 17: 145-149.
- Martyn, C., L. Illis, and J. Thom, Nabilone in the treatment of multiple sclerosis. The Lancet, 1995. 345: 579.
- Turcotte et al., Nabilone as an adjunctive to gabapentin
- Wissel, J., et al., Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain. Journal of Neurology, 2006. 253: 1337-1341.
- Notcutt et al, Initial experiences with medicinal extracts of cannabis
- Wade et al, A preliminary controlled study
- Corey-Bloom, J., et al., Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. Canadian Medical Association Journal, 2012. 184: 1143-1150.
- Greenberg, H.S., et al., Short‐term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clinical Pharmacology & Therapeutics, 1994. 55: 324-328.
- Collin, C., et al., Randomized controlled trial of cannabis‐based medicine in spasticity caused by multiple sclerosis. European Journal of Neurology, 2007. 14: 290-296.
- Collin, C., et al., A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurological research, 2010. 32: 451-459.
- Conte, A., et al., Cannabinoid‐induced effects on the nociceptive system: A neurophysiological study in patients with secondary progressive multiple sclerosis. European Journal of Pain, 2009. 13: 472-477.
- Kavia, R., et al., Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Multiple Sclerosis Journal, 2010. 16: 1349-1359.
- Langford, R., et al., A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. Journal of neurology, 2013. 260: 984-997.
- Leocani, L., et al., Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: a double-blind, placebo-controlled, crossover study. Neurol Sci, 2009. 30: 531‑4.
- Rog et al, Oromucosal δ9-
- The Mayo Clinic. Marijuana (cannabis sativa) - dosing
- Freeman, et al, The effect of cannabis on urge incontinence
- Killestein, et a.l, Immunomodulatory effects
- Vaney, et al., Efficacy, safety and tolerability of an orally administered cannabis extract
- Zajicek, et al., Multiple sclerosis and extract of cannabis
- Brady et al., An open-label pilot study of cannabis-based extracts
- Notcutt et al., Initial experiences with medicinal extracts of cannabis
- Wade et al, A preliminary controlled study
- The Mayo Clinic. Marijuana (cannabis sativa) - dosing
- Freeman et al., The effect of cannabis on urge incontinence in patients with multiple sclerosis
- Killestein, et a.l, Immunomodulatory effects
- Clifford, Tetrahydrocannabinol for tremor in multiple sclerosis
- Petro, D.J. and C. Ellenberger, Treatment of Human Spasticity with Δ9‐Tetrahydrocannabinol. The Journal of Clinical Pharmacology, 1981. 21(S1).
- Svendsen et al., Does the cannabinoid dronabinol reduce central pain?
- Ungerleider et al., Delta-9-THC in the treatment of spasticity
- The Mayo Clinic. Marijuana (cannabis sativa)) - dosing
- Brady et al., An open-label pilot study of cannabis-based extracts
- Turcotte et al., Nabilone as an adjunctive to gabapentin
- Wissel et al., Low dose treatment with the synthetic cannabinoid Nabilone
- Martyn et al, Nabilone in the treatment of multiple sclerosis
- Notcutt et al., Initial experiences with medicinal extracts of cannabis
- Wade et al, A preliminary controlled study
- Corey-Bloom et al., Smoked cannabis for spasticity in multiple sclerosis
- Corey-Bloom et al., Smoked cannabis for spasticity in multiple sclerosis
- The Mayo Clinic. Marijuana (cannabis sativa) - dosing
- Koppel et al, Systematic review
- Karst, M., S. Wippermann, and J. Ahrens, Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs, 2010. 70: 2409-38.
- Nielsen, S., et al., The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: A systematic review of reviews, 2017 (submitted)
- Shea, B.J., et al., Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology, 2007. 7: 1-7.
- Guyatt, G.H., et al., GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal, 2008. 336: 924-6.
- Johansen and Thomsen 2016, Review Article - Guidelines for Reporting Medical Research: A Critical Appraisal, International Scholarly Research Notices, article ID1346026

