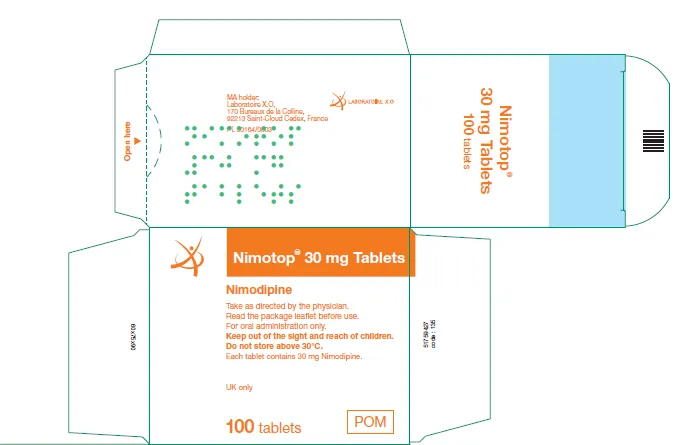
NIMOTOP nimodipine 30mg tablets (UK)
Section 19A approved medicine
NIMOTOP nimodipine 30mg tablets (UK)
Section 19A approval holder
Boucher & Muir Pty Ltd T/A Advanz Pharma ABN 58 000 140 474
Phone
1800 627 680
Approved until
Status
Current
Medicines in short supply/unavailable
NIMOTOP nimodipine 30 mg tablets blister pack - ARTG 43100
Indication(s)
Prophylaxis of ischaemic neurological deficits caused by cerebral vasospasm after subarachnoid haemorrhage following ruptured intracranial aneurysm
Images