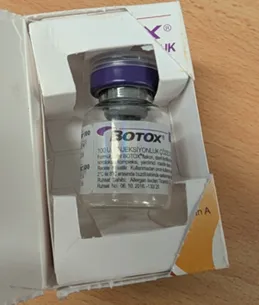
The Therapeutic Goods Administration (TGA) continues to see imports of counterfeit Botox vials despite a previous warning issued in July 2025.
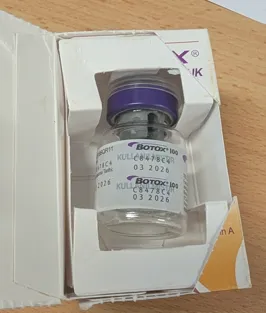
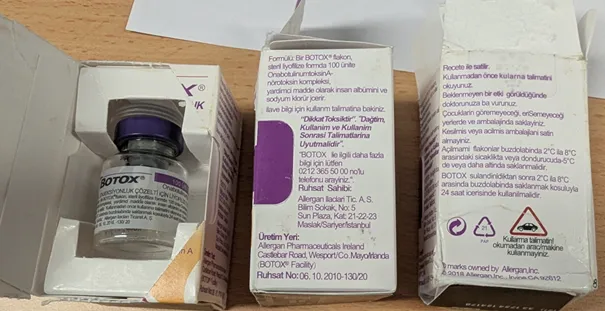
The counterfeit products were packaged to appear as a genuine Botox branded product manufactured by Allergan, an AbbVie company. However, AbbVie has confirmed these products, with batch number C8478C4, are not genuine.
Counterfeit products have not been assessed by us for quality, safety or efficacy. These products pose a significant public health and safety risk and should not be used.
As with the previous warning, these products were purchased online from an overseas website and not from an Australian pharmacy. Manufacturers of counterfeit goods are producing products that may appear legitimate. This highlights the importance of purchasing medicines from legitimate sources.
Botulinum toxin products are prescription-only medicines in Australia. While consumers, who hold a valid prescription, may lawfully import most prescription-only medicines for personal use, counterfeit products cannot be imported using the Personal Importation Scheme, under any circumstances, even with a valid prescription.
Importing, supplying and/or giving away counterfeit therapeutic goods is illegal.
We advise consumers to exercise extreme caution when buying medicines and medical devices online. For your safety, always buy medicines and medical devices from reputable sources and consult your healthcare provider or local registered pharmacy if you have any concerns.
Products purchased over the internet:
- may be fake
- may contain undisclosed and potentially harmful ingredients
- may not meet the same standards of quality, safety and efficacy as those approved by us for supply in Australia.
Information for consumers
- Counterfeit Allergan-branded Botox with batch numbers C8478C4 (identified in this alert) and C7211C4 and HA 33946 (identified in the July 2025 alert) should not be used. Take any remaining product to your local pharmacy for safe disposal.
- If you have any concerns arising from your use of this product, consult your health care practitioner.
- If you suspect you have had a side effect (also known as an adverse event) to this or a similar medicine, report it to the TGA via the adverse event reporting page.
- Where possible, keep the medicine, as we may request it for testing.
- If you are considering purchasing medicines from overseas, watch this short video on the risks associated with buying medicines and medical devices online.
Additional safety information for healthcare professionals
- Patients seeking cosmetic injectables are at increased risk of adverse events when injected with products that have not been approved for supply in Australia.
- Administering counterfeit products puts your patients at increased risk.
- Always check the product for any signs of counterfeiting before using it.
- Only the approved supplier (sponsor), as entered on the Australia Register of Therapeutic Goods (ARTG), or their authorised agent, can lawfully import cosmetic injectables into Australia for commercial supply. This includes the sale, administration, or application in treatment.
- Cosmetic injectables imported for commercial supply must be the version manufactured and approved for the Australian market, not a parallel import. These products must be entered on the ARTG under the supplier’s name prior to importation unless accessed lawfully via an approved pathway such as the Special Access Scheme (SAS).
- One of the known issues with parallel imports is that they may not be transported under appropriate cold chain storage protocols, which can compromise product viability. Internationally, this has been linked to acquired botulism infection as a severe adverse event following a cosmetic injection procedure.
Action we are taking
The TGA continues to check batch numbers of any products that may be subject to counterfeiting.
We will notify ABF to seize and destroy any of these counterfeit products intercepted at the border.
Report counterfeit medicines and medical devices
If you are worried about counterfeit medicines or medical devices, and want to report an issue, you can report the matter to the TGA:
| Phone: | 1800 020 653 |
| Online: | Report a problem or side effect |
| Email: | info@tga.gov.au |
Counterfeit Botox vials.
Imported product