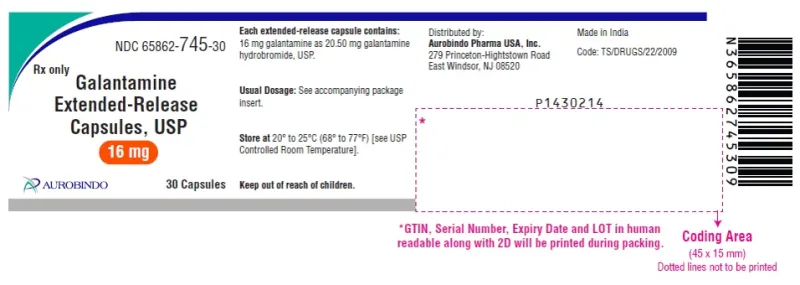
Galantamine Extended-Release Capsules, USP 16mg (Aurobindo, USA)
Section 19A approved medicine
Galantamine Extended-Release Capsules, USP 16mg (Aurobindo, USA)
Section 19A approval holder
Link Medical Products Pty Ltd ABN 73 010 971 517
Phone
1800 181 060
Approved until
Status
Current
Medicines in short supply/unavailable
APO-GALANTAMINE MR galantamine (as hydrobromide) 16 mg modified release capsules blister pack - ARTG 182041
GALANTYL galantamine (as hydrobromide) 16 mg modified release capsule blister pack - ARTG 157932
GAMINE XR galantamine (as hydrobromide) 16mg modified release capsules blister pack - ARTG 182038
REMINYL galantamine 16mg (as hydrobromide) modified release capsule blister pack - ARTG 97885
GALANTYL galantamine (as hydrobromide) 16 mg modified release capsule blister pack - ARTG 157932
GAMINE XR galantamine (as hydrobromide) 16mg modified release capsules blister pack - ARTG 182038
REMINYL galantamine 16mg (as hydrobromide) modified release capsule blister pack - ARTG 97885
Indication(s)
Galantamine is indicated for the treatment of mild to moderately severe dementia of the Alzheimer type.
Images