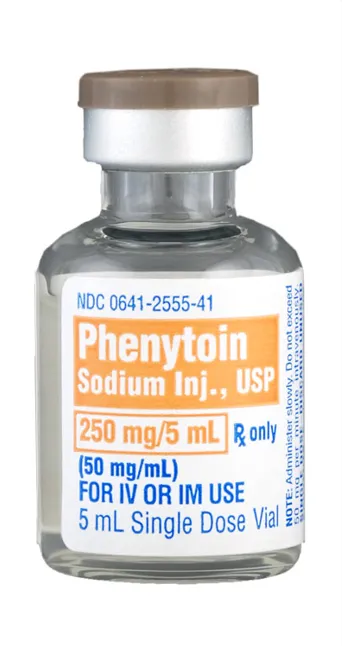
Phenytoin sodium injection 250mg/5mL single dose vial (Hikma, USA)
For the control of status epilepticus, tonic-clonic (grand mal) seizures and the prevention of seizures occurring during or following neurosurgery. Phenytoin will prevent or effectively decrease the incidence and severity of convulsive seizures in a high percentage of cases, with patients exhibiting little tendency to become resistant to its action. Besides its effectiveness in controlling seizures, phenytoin frequently improves the mental condition and outlook of epileptic patients.