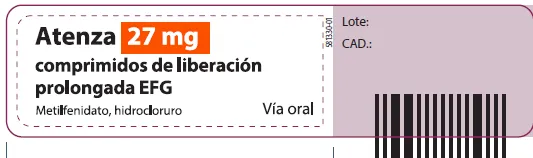
ATENZA methylphenidate hydrochloride 27mg prolonged-release tablets (Spain)
Section 19A approved medicine
ATENZA methylphenidate hydrochloride 27mg prolonged-release tablets (Spain)
Section 19A approval holder
Arrotex Pharmaceuticals Pty Ltd ABN 30 605 552 234
Phone
1800 195 055
Approved until
Status
Current
Medicines in short supply/unavailable
CONCERTA methylphenidate hydrochloride 27mg modified release tablet bottle - ARTG 124502
METHYLPHENIDATE-TEVA XR methylphenidate hydrochloride 27 mg modified release tablet bottle - ARTG 370911
METHYLPHENIDATE-TEVA XR methylphenidate hydrochloride 27 mg modified release tablet bottle - ARTG 370911
Indication(s)
Indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). Treatment should be commenced by a specialist.
Images