Description
Urolithin A is a synthetic version of urolithin A of the compound formed endogenously following consumption of ellagic acid and ellagitannins. Ellagitannins and ellagic acid are dietary polyphenols found in various fruits and berries (pomegranate, blackberries, camu-camu, strawberries, raspberries), nuts (walnuts, hazelnuts, acorns, chestnuts, pecans), muscadine grapes and oak-aged wines and spirits.
Urolithin A is manufactured via chemical synthesis:
- Chemical reaction between 2-bromo-5-hydroxybenzoic acid and resorcinol in the presence of sodium hydroxide and catalyst of cupric sulfate pentahydrate (copper (II) sulfate pentahydrate).
- Followed by protonation of the resulting di-or mono-sodium salt of Urolithin A and further purification by trituration in acetic acid.
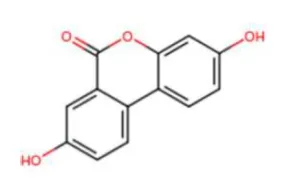
- Molecular formula: C13H8O4
- CAS number: 1143-70-0
- Molecular weight: 228.20
- Chemical names: 3,8-dihydroxy-6H-dibenzo(b,d)pyran-6-one; 3,8-dihydroxybenzo[c] chromen-6-one
- Chemical structure:
| Test | Method reference | Acceptance criteria[1] |
|---|---|---|
| Characterisation | ||
| General properties | ||
| Appearance | Visual inspection | Solid powder |
| Colour | Visual inspection | Beige to yellow |
| Identity | ||
| IR | FT-IR1 (USP<197>) | Observation of characteristic peaks at 3340, 3130,3090, 1700, 1615, 1470, 1340 cm-1. |
| NMR | 1H-NMR (USP<761>) | 1H-NMR: Observation of signals at 6.71-10.1 ppm. 13C-NMR: Observation of signals at 123.8-109.8 ppm. |
| MS | HPLC2-MS (USP<736>) | Observation of peak at retention time: 1.767 min |
| Assay | ||
| Assay – HPLC | HPLC2 | NLT 98.0% |
| Purity | ||
| Purity – HPLC | HPLC2 | NLT 98.5% |
| Water content | ||
| Water Content | Karl Fisher | NMT 1% |
| Impurities and incidental constituents | ||
| ||
| Acetic acid | GC3 | NMT 5000 ppm |
| ||
| Cd | Elemental Impurities-Procedures (USP-NF <233>) | NMT 0.5 ppm |
| Pb | NMT 0.5 ppm | |
| As | NMT 1.5 ppm | |
| Hg | NMT 0.1 ppm | |
| Cu | NMT 50 ppm | |
| Al | NMT 5 ppm | |
| Other heavy metals | NMT 1 ppm | |
| ||
3,11-dihydroxy-5H,9H-benzo[c]isochromeno[4,3-g]chromene-5,9-dione (AZX1) | HPLC2 | NMT 0.17% |
| Any other individual impurity | HPLC2 | NMT 0.17% |
| Residue on Ignition | USP<281> | NMT 0.5% |
Footnotes
FT IR KBr disc, wavenumbers: 4000-650 cm-1, resolution 4 cm-1, number of scan 16 method
HPLC Method: Column: Zorbax Eclipse Plus C18, 4.6 x 150 mm, 3.5 μm, Mobile Phase A: 0.05% TFA in H2O, Mobile Phase B: 0.05% TFA in ACN, Flow Rate 1.2 ml/min, Detection: VWD: 230 nm, Column Temperature: 40°C, Injection Volume: 7 μL, Acquisition Time: 28.0 minutes (including 8.0 minutes re-equilibration)
GC Method: Column: Restek Rtx-624 30 m x 0.32 mm, 1.8 μm film or equivalent, Injector. Mode: Constant Flow, Flow: 3.0 mL/min; Inlet: Injection Volume: 1.0μL, Mode: Split, Split Ratio: 50, Temperature 180°C, Carrier Gas: helium; Oven Ramp: Initial Temperature: 40°C, hold 2 minutes, Ramp: 10°C/minute to 220°C, hold 3 minutes., Run Time: 23 minutes: Detector: Temperature: 280°C, Air Flow: 350 mL/min, Hydrogen: 40 mL/min, Makeup Flow: 40 mL/min (combined). Approximate RT acetic acid = 6.7 minutes.
Abbreviations
- ACN: Acetonitrile
- CAS: Chemical Abstract Services
- FT IR: Fourier transformed Infrared Spectroscopy
- GC: Gas Chromatography
- HPLC: High-Pressure liquid Chromatography
- MS: Mass Spectrophotometry
- RT: Retention Time
- TFA: Trifluoroacetic Acid
- USP-NF: United States Pharmacopeia and the National Formulary

