We will have limited operations from 15:00 Wednesday 24 December 2025 (AEDT) until Friday 2 January 2026. Find out how to contact us during the holiday period.
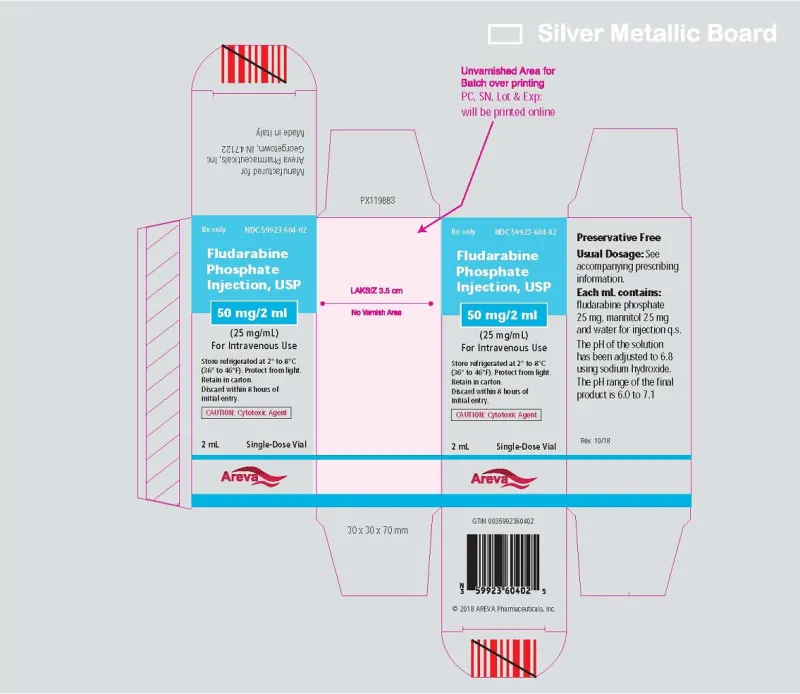
Fludarabine Phosphate Injection, USP 50 mg/2mL (25 mg/mL) Single-Dose Vial (Areva, USA)
Section 19A approved medicine
Fludarabine Phosphate Injection, USP 50 mg/2mL (25 mg/mL) Single-Dose Vial (Areva, USA)
Section 19A approval holder
Pro Pharmaceuticals Group ABN 20 605 457 430
Phone
1300 077 674
Approved until
Status
Current
Medicines in short supply/unavailable
FLUDARABINE JUNO fludarabine phosphate 50 mg powder for injection vial - ARTG 147831
Indication(s)
Treatment of B-cell chronic lymphocytic leukaemia.
Images