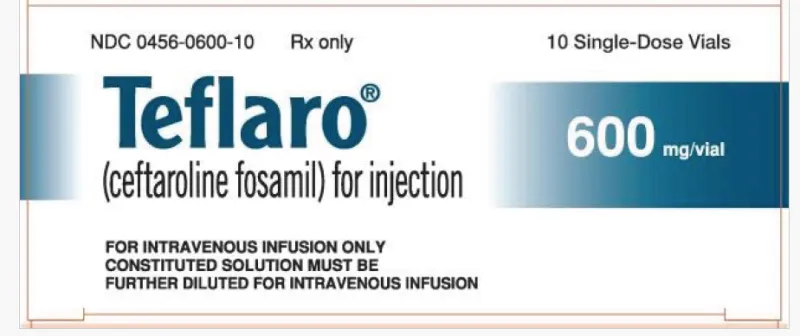
TEFLARO ceftaroline fosamil for injection 600mg single-dose vial (USA)
Section 19A approved medicine
TEFLARO ceftaroline fosamil for injection 600mg single-dose vial (USA)
Section 19A approval holder
Orspec Pharma Pty Ltd ABN 15 634 980 417
Phone
02 4339 4239
Approved until
Status
Current
Medicines in short supply/unavailable
ZINFORO ceftaroline fosamil 600mg powder for injection vial - ARTG 192260
Indication(s)
The treatment of patients with the following infections proven or strongly suspected to be caused by designated susceptible bacteria:
- Complicated skin and soft tissue infections (cSSTI).
- Community-acquired pneumonia (CAP).
Images